- Volume 65 , Number 1
- Page: 95–7
Leprosy in children
This department is for the publication of informal communications that arc of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this JOURNAL is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the JOURNAL and thus interfere with its prime purpose.
To the Editor:
Although the incidence of leprosy is declining, it is still a public health problem in Malaysia. The 1994 Annual Report from the National Leprosy Control showed on page 12 that the prevalence rate per 10,000 in the entire nation had reduced from 3.4 in the year 1988 to 1.7 in the year 1994. The state of Sabah in Fast Malaysia is the biggest state in Malaysia. The prevalence rate of leprosy per 10,000 was 2.9 in 1988 and 2.0 in 1994 in Sabah, indicating a slow decline. Papers on leprosy from Malaysia based on therapy, immunology and autopsy findings (6,7,15) have been published in the past. The aim of this paper is to highlight the clinical and pathological features of leprosy in Malaysian children from the state of Sabah.
Skin biopsies from patients attending dermatology clinics in general hospitals in Sabah who were suspected to have leprosyhave been sent to the Department of Pathology, University Hospital, since January1994. Formalin-fixed, paraffin-embedded sections were stained with hematoxylin andeosin (H&E) and Fite's stain for acid-fast bacilli (AFB). The histological classification of leprosy was based on the Ridley-Jopling system (8).
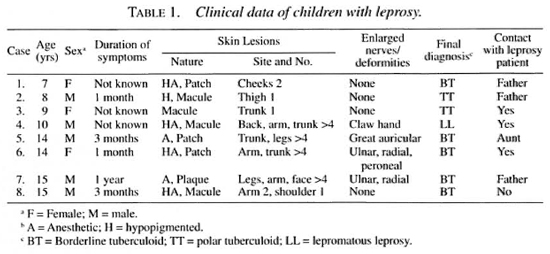
A total of 87 skin biopsies from 71 leprosy patients were received between January 1994 and December 1995. There were 8 children among the 54 newly diagnosed leprosy patients, 5 boys and 3 girls. The age range of these patients was 7 to 15 years, and the duration of symptoms varied from 1 month to 1 year. Seven children gave a history of contact with a leprosy patient. Two of the patients had a single lesion, while the others had two or more lesions at the time of presentation. The nature of the lesionwas macule (4 cases), patch (3 cases) and plaque ( I case), hypopigmented (5 cases) and anesthetic (5 cases). Although thickened peripheral nerves were observed inthree patients, only one had claw-hand deformity. None of the patients had testicularatrophy or gynecomastia or other evidence of systemic involvement. The clinical dataof patients is given in Table 1.
The histology of the skin biopsies in two cases showed features of polar tuberculoid leprosy (TT), such as epithelioid granuloma with numerous lymphocytes, and evidence of hypersensitivity reaction such as extensive erosion of the epidermis by lymphocytes (one case) and numerous giant cells of the Langhans' type (one case). In five cases, the histology was that of borderline tuberculoid (BT) leprosy and AFB stainingshowed an occasional bacillus.
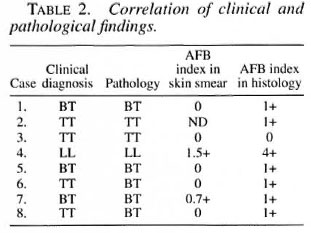
There was no case of intermediate leprosy. Slit-skin smears were done in sevencases of tuberculoid leprosy and were nega-tive in five cases (Table 2). Histology showed AFB in seven cases, although the AFB were negative in the skin smears offive cases. The correlation between histology and clinical features is 62.5%.
The patients were started on multidrug therapy (MDT). Two patients defaulted. Case 5 developed drug-induced jaundice and fever 4 weeks after commencing the therapy. One patient with LL developed fever and swelling of the existing skin lesions. A biopsy showed epithelioid cell granuloma with lymphocytes and a few bacilli, features of BT.
Children composed 14% (8/52) of newly detected leprosy cases in this 2-year study. The duration of symptoms was less than 1 year, and 87% of the patients had tuberculoid leprosy. The common location of skin lesions was on the extremities; such observations are similar to those noted by Sehgal, et al. (9,12,14) . The youngest patient in our study was 7 years old. Although the incubation period of leprosy is believed to be years, it has been reported in infants as early as 2 months of age (2). The age of onset of leprosy varies in different countries (3). It is known that children form a highrisk group in families of leprosy patients. Dave and Agarwal (3) studied children in 200 families of which 100 families had a family member with active leprosy. On comparing these two groups, the prevalence rate was 14.2 times higher among the contacts. Although the total number of patients in our study is small, 7 out of 8 patients had a history of contact with a leprosy patient.
We recommend that family members of newly diagnosed patients be screened regularly for leprosy. This would allow earlier institution of therapy and reduce morbidity and deformity. The skin lesions in children are usually single or few because the majority of them suffer from indeterminate or tuberculoid leprosy (13). The lesions were hypopigmented (five cases) and anesthetic (six cases). The differential diagnosis of hypopigmented patches in children include pityriasis alba, pityriasis versicolor, vitiligo and leprosy (5). There was a reasonably good correlation between clinical and histological findings in 62.5% of our cases compared to that reported by Sehgal (l4) where such correlation was present in only 56% of the cases. Histopathologic changes suggestive of indeterminate leprosy were noted in 4% of cases studied by Sehgal, et al. (12); in our study, histology showed well-formed granulomas in all cases. Clinical, bacteriological, histopathological and immunological features should be considered while diagnosing leprosy in children. In the present study, the AFB positivity in slit-skin smear and histological sections was 16.6% and 91.6% of cases, respectively. This indicates that slit-skin smear examination is not a good method for the diagnosis of leprosy in children. Reactional episodes (4,11) and disabilities (15) are rare in children. We have noted upgrading reaction and claw-hand deformity in only one case so far.
- Pailoor Jayalakshmi, M.Path.,
M.R.C. Path.
Associate Professor
Department of Pathology
Faculty of Medicine
University of Malaya
50603 Kuala Lumpur, Malaysia
(Member, Leprosy Research Committee)
- M.Tong, M.R.C.P.
Consultant Dermatologist
- Santokh Sing, M.P.H.
Senior Health Officer
Leprosy Control Program
Queen Elizabeth Hospital
Sabah, Malaysia
- T. Ganesapillai, F.R.A.C.P, F.A.A.D. (U.S.A.)
Chief Consultant Dermatologist
General Hospital
Kuala Lumpur, Malaysia
(Technical Advisor, Leprosy Research Committee)
REFERENCES
1. BADGER, L. F. Epidemiology. In: Leprosy in Theory and Practice. 2nd edn. Cochrane. R. G. and Davey, T. F., eds. Bristol: John Wright & Sons. Ltd., 1964, pp. 69-97.
2. BRUBAKER, M. L., MEYERS, W. M. and BOURLAND, J. Leprosy in children one year of age and under. Int. J. Lepr. 53(1985)517-523.
3. DAVE, D. S. and AGARWAL, S. K. Prevalence of leprosy in children of leprosy patients. Indian J. Lepr. 56(1984)615-621.
4. DEBI, B. and MOHANTHI, H. C. Reactional states of leprosy: a clinical assessment. Lepr. India 49(1977)229-233.
5. DUTTA, R. K. and MURTHY, N. A study of hypopigmented lesions in leprosy. Lepr. India 53(1981)634-640.
6. GAN, S. C. Phenolic glycolipid antigenemia in leprosy. Mai. J. Med. Lab. Sci. 9(1992)109-112.
7. JAYALAKSHMI, P., LOOI, L. M., LIM, K. J. and RAJAGOPALAN, K. Autopsy findings in 35 cases of leprosy in Malaysia. Int. J. Lepr. 55(1987)510-514.
8. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
9. SEHGAL, V. N. and CHAUDHRY, A. K. Leprosy in children: a prospective study. Int. J. Dermatol. 32(1993)194-197.
10. SEHGAL, V. N. and JOGINDER. Leprosy in children: correlation of clinical, histopathological, bacteriological and immunological parameters. Lepr. Rev. 60(1989)202-205.
11. SEHGAL, V. N., REGE, V. L. and MASCARENHAS, M. F. Pattern of reactions in leprosy: a clinical appraisal. Lepr. India 49(1977)221-228.
12. SEHGAL, V. N., REGE, V. L. MASCARENHAS, M. F. and REYE, M. The prevalence of leprosy in a school survey. Int. J. Lepr. 45(1977)360-363.
13. SEHGAL, V. N.. REGE, V. L. and REYE, M. Correlation between clinical and histopathologic classification in leprosy. Int. J. Lepr. 45(1977)278-280.
14. SEHGAL, V. N. and SRIVATHSAVA. G. Leprosy in children. Int. J. Dermatol. 26(1987)557-566.
15. WATERS, M. F. R., REES, R. J. W., PEARSON, J. M. H., LIANG, A. B. G.. HELMY, H.S. and GELBER, R. H. Rifampicin for lepromatous leprosy: nine years experience. Br. Med. J. 1(1978)133-136.