- Volume 65 , Number 2
- Page: 170–7
Cervical branch of the facial nerve in leprosy
ABSTRACT
This study demonstrates that the platysma is occasionally palsied in leprosy and that this only occurs when the facial nerve already has some other palsy. That there needs to be a facial palsy before there can be a platysma palsy is strongly suggested, in that there was no case of an isolated platysma palsy. Patients, regardless of age or other factors, could mimic a platysma contraction. This obviates the need for electrical testing to examine for a platysma palsy. It also means that a nonfunctioning platysma on clinical examination is, in fact, a palsied platysma. While lagophthalmos is regularly examined for, and any obvious facial paresis would be noticed, less severe forms of facial muscle paresis will only be found if formally examined for. The mechanism whereby the facial nerve is involved in leprosy is not clarified, but our findings suggest that proximal spread of a lesion that began in the zygomatico-temporal branches and reaches to the facial nerve trunk is more likely than new lesions developing de novo in other peripheral facial nerve branches. That the primary lesion is within the facial nerve trunk in all cases but we only see the frequent zygomatic sequelae due to secondary factors is not excluded.RÉSUMÉ
Cette étude démontre que le platysma est parfois paralysé dans la lèpre, et que ceci survient quand le nerf facial a déjà une autre paralysie. QiCil y ait besoin d'avoir une paralysie faciale avant qu'il puisse y avoir une paralysie du platysma est fortement suggéré par le fait qu'il n'y avait aucun cas de paralysie isolée du platysma. Des patients, quel que soit leur âge ou d'autres facteurs, pouvaient imiter une contraction du platysma. Ceci montre le besoin d'un test électrique pour rechercher une paralysie du platysma. Ceci signifie également qu'un platysma non fonctionnel à l'examen clinique est, en fait, un platysma paralysé. Alors qu'on recherche régulièrement l'existence d'un lagophtalmos, et que des paralysies faciales évidentes seront notées, des formes moins sévères de parésie du muscle facial seront trouvées seulement si on les recherche formellement. Le mécanisme par lequel le nerf facial est impliqué dans la lèpre n'est pas clarifié, mais nos observations suggèrent que la dissémination de proche en proche d'une lésion qui a commencé dans les branches zygomatico-temporelles et atteint le tronc du nerf facial est plus vraisemblable que des nouvelles lésions se développant de novo dans d'autres branches périphériques du nerf facial. Il n'est pas exclu que la lésion primaire ne soit dans tous les cas dans le tronc du nerf facial, mais que nous ne voyions les séquelles zygomatiques fréquentes que suite à des facteurs secondaires.RESUMEN
Este estudio demuestra que los músculos de la platisma en los pacientes con lepra pueden ocasionalmente sufrir parálisis y que esto sólo ocurre cuando el nervio facial tiene ya alguna otra parálisis. La idea de que se retiñiere de una parálisis facial preexistente para que ocurra parálisis en la platisma deriva del hecho de que no se ha reportado un sólo caso de parálisis aislada de la platisma. Los pacientes, independientemente delà edad o de otros factores, pueden inducirse a contraer la platisma. Esto obvia la necesidad de las pruebas eléctricas para examinar una posible parálisis de la platisma. Una platisma no funcional al examen clínico es, de hecho, una platisma paralizada. Mientras que regularmente se hace el examen para lagoftalmos y cualquier paresis facial obvia es fácilmente notada, las formas menos severas de las paresias faciales sólo pueden distinguirse si el examen se hace en forma dirigida. El mecanismo por el cual se afecta el nervio facial en la lepra no está bien aclarado pero nuestros resultados sugieren que la dispersión próxima! de una lesión que comienza en las ramas zigomático-temporal y alcanza el tronco nervioso del nervio facial, es más probable que la aparición de novo de lesiones en otras ramas periféricas del nervio facial. No se exluye la posibilidad de que la lesión primaria esté dentro del tronco del nervio facial en todos los casos y que sólo veamos las secuelas sigomáticas debido a otros factores secundarios.The pathogenesis of leprosy is poorly understood. The present understanding is that leprosy bacilli gain entrance to nerves (by methods unknown) and are phagocytosed by Schwann cells. A pattern of nerve involvement is recognized, with certain "sites of predilection" being rendered preferentially nonfunctional leading to recognized impairment patterns. There is considerable debate as to whether the nerve is primarily damaged at these sites of predilection or whether the nerve is involved more distally and/or proximally, but because of the mechanical problems of the sites of predilection any long-term compression will lead to irreversible nerve damage at these sites.
Also, the involved nerves are often associated with a nearby skin patch. Whether the bacteria gain access to the nerve by the blood stream, as evidenced by its segmental and longitudinal fascicular nature, or by a local skin lesion, as evidenced by the association with skin patches, is not known (21).
The prevalence of facial nerve involvement is 4%-10% of all cases of leprosy (4,14,15,19,22 ) . The facial nerve differs From the other nerves involved in leprosy because it is a pure motor nerve (in its extra cranial portion). Motor loss From a facial nerve lesion is usually restricted to temporo-zygomatic branches where they cross the zygomatic arch, leading to orbicularis oculi paralysis. That the zygomatico-temporal branches are more frequently involved than other branches is attributed to the double fascia entrapment overlying the zygomatic arch (7,8) and to their superficial location. Possibly other secondary factors, such as the colder temperature, increased stretch and the increased likelihood of trauma at this site, are relevant.
However, this has been challenged by Dastur, el al. (2,8) with their study comparing facial nerve involvement with trigeminal nerve malar skin patches and suggesting that there is retrograde motor branch infection with the leprosy bacilli and, thus, facial palsy. That the upper part of the face is more commonly involved than the lower is noted, (3,4,7,8,14,15,18,19,22,28) but not proven. That the nerve is involved more distally than proximally is not proven but assumed, in that palsies are more frequent in the zygomatic region. The recorded incidence of facial nerve involvement is based on screening examinations for weakness of eye closure because of the sequelae of lagophthalmos and possible loss of vision. In most leprosy clinics there is no routine testing of all of the facial nerve's branches.
You might expect the cervical branch of the facial nerve to be involved frequently since it innervates a panniculus carnosus muscle, with its muscle fibers inserted superficially into the dermis. It is also surrounded by sensory cutaneous neck nerves which are frequently involved. However, most leprologists do not regularly examine the action of the platysma since any functional deficit would be of little clinical relevance. Some believe the cervical branch never to be involved (6) while we at Green Pastures Hospital (GPH) have noticed it to be occasionally involved in those with a facial nerve palsy.
The classical understanding remains unproven and challenged by several issues. Leprosy nerve pathology is segmental and has an uneven involvement of fascicles. Recent intra-operative electrodiagnostics to find the most proximal lesion in the median and ulnar nerves has demonstrated lesions far more proximal than the naked eye can perceive (26, 27). The phenomenon of misreinnervation of the facial musculature (synkinesis) (l8) also suggests that the pathology is often more proximal than just a named terminal nerve branch; perhaps as far as the division of the main facial nerve trunk, allowing regenerating axons to be able to misreinnervate a nerve fascicle. Most patients with some degree of facial muscle weakness also have evidence of mis-reinnervation, the commonest being elevation of the upper lip on gentle eye closure. The platysma is an extremely superficial muscle, and the cervical branch of the facial nerve is known to have anastomotic connections with the transverse cutaneous cervical nerve (29). For these reasons we might expect it to be involved more frequently. However, the cervical branch of the facial nerve is deep to the platysma muscle, and because of its anatomical site would be protected From secondary factors.
We wanted to know whether or not the cervical branch of the facial nerve was involved in leprosy. And if involved, what was the relationship with any pre-existing facial nerve lesion or enlarged cervical sensory nerves?
SUBJECTS AND METHODS
One-hundred-two consecutive leprosy patients attending Green Pastures Hospital over a period of 3 months were examined according to the protocol. Seven patients requesting reconstruction of a facial deformity caused by a near total facial nerve palsy also were examined.
For each patient, their name, age, sex, type of leprosy, treatment nature, and stage was recorded. A note was made of any reaction, and whether the patient was taking corticosteroids at the time. The patient was asked if there had been any ear discharge in the last 12 months (which would indicate a possibility of middle ear disease, providing an alternative explanation for a facial nerve lesion). Consenting patients then had a further clinical examination of the facial nerve's five main peripheral branches by asking for the voluntary contraction of each muscle group. In vivo facial nerve anatomy is not stylized to the five branches described in anatomy textbooks but, rather, has several interfascicular interconnections, and no one named branch is responsible for one facial expression.
We tested as follows: temporal branch by elevation of the frontalis muscle, zygomatic branch by eyelid closure, buccal branch by holding air in the buccal pouch against resistance and by the ability to elevate the angle of the mouth, and the cervical branch by contracting the platysma. The mandibular branch, with its connection to the infra-orbital buccal plexus was tested by the "buccal" branch tests and not formally by any depressor actions of the orbicularis muscles. Each action was recorded as being normal or weak. In order to examine the facial nerve it is usual to teach patients to carry out movements on instruction, or to mimic actions. Such as, "smile," "close your eyes," "blow out your cheeks" and "frown." The platysma, however, is more difficult because previously we did not examine for its function and because there is no command to initiate it, so it has to be a mimicked action. We were unsure whether patients would correctly mimic the platysma action, leaving us with a result where a nonfunctioning platysma might mean that there was no cervical branch activity or simply a failure in the patient's comprehension.
To overcome this and to compare any nonfunctioning platysma with branches of the facial nerve that we know we can examine clinically, we decided to test the frontalis, buccal and platysma muscles using electrical stimulation delivered by an eutrophic (10,11) muscle stimulator (Dynamic Medical Instruments). Current was delivered via 2 cm2 carbon rubber electrodes with the active electrode being placed on the motor point of the relevant muscle. The stimulator delivers 80 microsecond compensated rectangular pulses at between 0 and 18 volts. Each was recorded as being positive or negative, depending on whether a contraction could be stimulated before the maximum output was reached or not, or before it became too painful.
Also, because some patients may have had a platysma palsy which was partial or recent, the function of the cervical branch of the facial nerve was recorded electrophysiologically using strength-duration (12) curves in all patients. These indicate the strength of impulses of various durations required to produce visible contraction in the muscle. A Myodyne stimulator (Electro-Medical Supplies) of the constant voltage type was used to supply rectangular impulses of different durations varying From 300 ms to 1 ms via a 2 cm2 carbon rubber electrode and a button electrode.
The patient was seated comfortably in good light, and the skin resistance was reduced by cleansing with an alcohol swab. The indifferent electrode (anode) was applied to the anterior aspect of the neck in the midline, and the active electrode (cathode) was applied to the motor point with care being taken to keep it over the same point throughout the test. Current was applied using the longest stimulus first (300 ms), and the voltage was increased until the minimum observable contraction was obtained. The strength-duration curve was plotted, and each patient was classified as innervated, denervated or partially innervated on the basis of the shape of the graph (12).
Corneal and maxillary sensation were tested using cotton wisps, and a skin patch was looked for on the face. If a recent history of ear discharge was elicited, the drums were checked and recorded as intact or perforated.
RESULTS
Of the 102 patients examined, 96 had a complete record for computerized statistical analysis. Patients were generally middle aged (median age 44 years, range 11-77), 76 were male (79%) and 20 female (21%). The patients had been classified as 46% borderline tuberculoid (BT), 28% borderline lepromatous (BL), 15% lepromatous (LL), 6% borderline (BB), 3.3% pure neural (PN) and 1% tuberculoid (TT). Of the 96 patients, 52 (54%) had completed multidrug therapy, 44 (46%) were on therapy, and no patient was new. As regards leprosy reactions in the previous 12 months, 69 (73%) of the patients had had none, 20 (21 %) had had a type 1 or reversal reaction and 6 (6%) had had a type 2 or erythema nodosum leprosum (ENL) reaction. Nineteen patients (20%) were on corticosteroids.
For each patient there are two records of facial nerve examination, one for each side, right and left. Of 192 nerves (96 patients) only one platysma was found to be paralyzed. This was a right-sided palsy and was associated with a near total palsy in the other branches of the facial nerve. No patient was found to have an isolated cervical branch nerve palsy on voluntary contraction or eutrophic stimulation. No patient had a denervated or partially denervated cervical branch of the facial nerve by strength-duration curves.
The strength-duration curves were validated for denervation for one of our patients (PS), in that 2 weeks after surgery to excise her facial nerve and reconstruct her right facial palsy, she had a denervated curve on the right and a normal one on the left. No patient was unable voluntarily to contract their platysma. Twenty-five patients had some form of facial nerve palsy; 9 were unilateral and 16 bilateral.
Statistical analysis of the data did not establish a relationship between loss of maxillary sensation, enlargement of cutaneous neck nerves, or the presence of face patches with any pattern of facial nerve palsy. Twenty-six of the patients had had a reaction in the previous 12 months, and 19 were on corticosteroids but only 4 had any facial nerve involvement. The study questionnaire only inquired if there had been a reaction in the past 12 months and, thus, any relationship between a previous reaction and facial nerve palsy was not possible to establish From these data. Two patients had an ear discharge, one with no facial nerve lesion and one with a bilateral lagophthalmos but no other facial nerve involvement. There was no evidence to suggest that a facial nerve palsy was associated with middle ear disease.
Patients referred to the surgical assessment clinic for facial nerve problems requiring more than simple tarsorrhaphy were also examined for platysma function. Their clinical details are summarized in Table 1. Most of these patients had a surgical procedure to help relieve their impairment and, at the same time, a biopsy of portions of their nonfunctioning facial nerve. A biopsy of the cervical branch of the facial nerve was also done regardless of whether there was a platysma palsy or not. (The results of this work will be the subject of another paper).
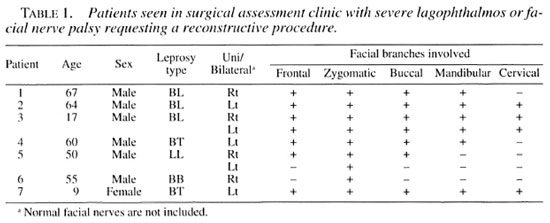
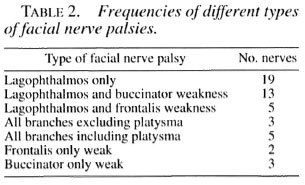
Combining all of the patients with any degree of facial palsy, i.e., 25 From the consecutive examinations and 7 From the surgical assessment clinic, a total of 32 patients with 50 involved facial nerves were available for analysis. The frequency of named nerve branches involved alone, or in association with other involved facial nerve branches, making up the different patterns of facial nerve palsies can be seen in Table 2.
DISCUSSION
This study demonstrates that the platysma is occasionally palsied in leprosy, and that in our sample this only occurred when the facial nerve already had some other palsy. This is the first study which has actually examined the status of the platysma, and it certainly refutes the claim that it is never involved (6). There was no case of an isolated platysma palsy, confirming that there needs to be a facial palsy before there can be a platysma palsy. To prove this statement, however, would need the screening of a very large number of patients (1000 perhaps). Patients without lagophthalmos or any degree of eye weakness would need to be examined for facial nerve involvement, and this is now a possibility since we know From this study that all patients regardless of age or other factors can easily mimic this platysma contraction. This obviates the need for electrical testing to examine for a platysma palsy. It also means that a nonfunctioning platysma on clinical examination is, in fact, a palsied platysma. While lagophthalmos is regularly examined for, and an obvious facial paresis would be noticed, less severe forms of facial muscle paresis will only be found if formally examined for.
The facial nerve once it has come through the mastoid foramen has only motor branches. Some of these are deep, such as the posterior auricular nerve which becomes the auricular branch to the intrinsic muscles of the external ear, and the occipital branch, which supplies the occipital belly of the occipitofrontalis. There is also a digastric branch for the posterior belly of the digastric, and stylohyoid branch for the middle part of the stylohyoid muscle. None of these muscles are easy to examine for a palsy, and we are not aware of any reported occurrences. It is of interest that patient no. 3, who had a bilateral platysma palsy, did have one small muscle belly (on the right only) that contracted under the chin in a superior-inferior direction when voluntarily trying to contract his platysma. This could be a cervical branch innervated strap muscle, suggesting mis-reinnervation of the cervical nerve, or it is his stylohyoid muscle which is innervated by the stylohyoid branch of the facial nerve.
The more well-known branches of the facial nerve (frontal, temporal, zygomatic, buccal, marginal mandibular, and cervical) all run deep to the superficial lobe of the parotid gland and superficial muscular and aponeurotic system (SMAS) before entering their respective muscles on their deep surface (29).
The problem with any facial nerve palsy is to determine the site of the nerve lesion. If it is a lower motor neuron type, then where anatomically is it damaged and by what? In leprosy we have always thought that the nerve damage is distal and at the sites of predilection. We believe that the mycobacterium is in the nerve at the level of the lesion and that the ensuing pathology is a direct result of the destructive nature of this local infection and any local and systemic immunological reaction. The distribution of the disease to certain portions of peripheral nerves is attributed to: a) the anatomical position permitting a relative superficiality and, therefore, coolness for which the mycobacteria are said to have an affinity (20), and b) the anatomical position causing the nerve to pass through a fibroosseus tunnel at these sites, making it unable to avoid compression when it becomes swollen in an inflammatory reaction. Which of these two reasons is the more important is not known. Is the relative coolness due to superficiality the primary cause for M. leprae infection in that portion of the nerve? Or is the leprosy bacterium's affinity for nerves more generalized than we think, and much more of a peripheral nerve trunk is infected but only the swollen portion at the "site of predilection" undergoes compression and presents the clinical picture of neuritis that is recognized by the clinician?
There is now evidence to suggest that nerve involvement in the peripheral nerve trunks, can be more proximal. Turkof, et al. have demonstrated electrophysiological^ other involvements in the peripheral nerve trunks at levels much higher than 'previously noticed macroscopically. These are not at sites of compression, and after neurolysis there was some functional improvement (26-27). A post-mortem study on three lepromatous patients has found some immunohistochemical evidence of facial nerve involvement within the internal auditory meatal and tympanic portions of the temporal bone, but no nerve damage was observed (16).
There is also considerable debate as to whether the infection begins in the dermis and spreads centripetally, ascending along the nerve, or whether it is a systemic disease due to hematogenous infection. The resulting lesions of a systemic disease could be the result of disseminated infective material or immunological reactions to the infective agent's antigens. To support the ascending infection theory, there is Miko, et al. 's anatomical and pathological study of amputated limbs From leprosy patients (17). They serially examined the nerves From proximal nerve trunks down to distal dermal nerve endings, and demonstrated increasing nerve destruction the more distally the nerve was examined, culminating in total destruction of dermal nerves and sensory nerve endings. A recent report of armadillos infected intravenously with M. leprae showed extensive involvement of the peripheral nerves, increasing in intensity as the nerve was followed distally in those animals that had developed disseminated disease (23). In support of a systemic disease theory there is: a) the production of skin patches typical of nonlepromatous leprosy in an inbred strain of guinea pig as the result of an autoimmnune response to an injected sensory nerve antigen (5,6,24,25) the pure neural (PN) classification of leprosy where patients have the typical neuritis but are never shown to have a skin lesion; c) patients in reversal reaction frequently get a worsening neuritis at the sites of predilection but not associated with a nearby skin patch in reaction whereas facial nerve palsy often has a history of a type 1 reaction in a nearby malar skin patch at the same time as the development of the palsy.
Dastur, et al. were the first to suggest a retrograde infection of the zygomatic branches of the facial nerve From a concomitant trigeminal facial skin patch infection. There is a known interconnection From the zygomaticofacial branch of the maxillary division of the trigeminal nerve with the zygomatic branches of the facial nerve. Based on a study of 11 cases, Dastur, et al. hypothesized that the leprous infection enters the malar skin through sensory nerve terminals of the trigeminal nerve and progresses in such a way as to involve the motor fibers of the facial nerve in this area, either because of the close proximity to, or the actual anastomotic connections between the palpebral branches of the maxillary division of the trigeminal nerve and the zygomatic branches of the facial nerve (8). Other studies also have supported the idea that the facial skin patch was causative for the subsequent facial nerve palsy (13,19). Hogeweg, et al. .showed that a malar skin patch in type 1 reaction was a precondition for facial nerve damage on the same side (13). Patients with lagophthalmos often have hypopigmented anesthetic malar patches (2,19).
Secondary factors operating on the nerve branches in the zygomatic region are also thought to be important. Dastur, et al. were impressed by the close apposition of these branches against the zygoma and considered compression against unyielding fibroosseous tissues at this site a "mechanical predisposition." Their study also showed that the nerves are not thickened (8). Lubbers, et al. looked at 57 patients with lagophthalmus to assess the degree of paralysis of facial muscles and found that most (81 %) had involvement of at least one other muscle group. Upper and lower facial muscles were found to be affected in the same proportion and so they found little support for the statement that the superficial course of the facial nerve above the zygomatic bone is decisive for exclusive paralysis of the zygomatic branch (14).
However, many other facial nerve motor branches have similar anastomotic connections with cutaneous sensory nerves, including the postauricular and the cervical. The facial nerve also has an ill-defined area of sensory innervation of the external ear (29), and perhaps the facial nerve lesion is direct bacterial invasion From this skin source into the facial nerve's cutaneous sensory branch. Cutaneous nerves in the neck are one of the most commonly enlarged nerves to be found in leprosy, yet our study has not found the platysma to be as commonly paralyzed. In fact, it is only involved when part of a more serious facial nerve lesion. This observation, together with the phenomenon of mis-reinnervation, suggests that cervical branch involvement only occurs as facial nerve pathology progresses more proximally From its zygomatic position toward the trunk. Or it could be that the trunk is always involved and we only see the lagophthalmos problem more commonly than other branch involvement because of all the secondary factors of compression at the zygoma (SMAS and deep investing cervical fascia fixed to the periosteum over the zygoma), cooler temperature and exposure to trauma. Another superficial motor nerve in the neck is the accessory nerve supplying the trapezius muscle. There is no report of this muscle being weak or paralyzed in leprosy, even though it is situated superficially just under the skin in the posterior triangle of the neck, and often surrounded by many enlarged cutaneous sensory nerves. Evidence of its superficiality is the frequency of inadvertent damage in nonleprosy patients having a biopsy of a lesion in the posterior triangle of the neck (1,19), and the patient presenting later with a complaint of muscle weakness.
Of the facial nerve innervated facial muscles, the orbicularis oculi is the muscle most frequently involved alone by leprosy (Table 2). Then an orbicularis oculi weakness together with the buccinator or, less frequently, with the frontalis, is the next most likely finding. The low frequency of a palsy of all branches excluding the cervical, and then including the cervical, implies a worsening nerve scenario progressing proximally along the facial nerve trunk rather than increasing frequencies of skin acquired nerve involvement.
Acknowledgment. We thank Dr. Alison Anderson (statistician) for her hours of help in getting the data onto a computer program (Epi-Info 6.1). We also thank Dr. Frauke Woepcl for allowing us to use the facilities of Green Pastures Hospital, and we thank all the patients for their quiet endurance of us and our examination of them.
REFERENCES
1. ALNOT, J. L., ABOUJAOUDE, J. and OBERLIN, C. Traumatic lesions of the spinal accessory nerve. II. Clinical study and results of a series of 25 cases. Rev. Chir. Orthop. Réparatrice Appar. Mot. 80 (1994)297-304.
2. ANTIA, N. IL. DIVEKAR, S. C. and DASTUR, D. K. The facial nerve in leprosy. I. Clinical and operative aspects. Int. J. Lepr. 34 (1966) 103-117.
3. CHACO, J., MAGORA, A., ZAUBERMAN, H. and LANDAU, Y. An electromyographic study of lagophthalmos in leprosy. Int. J. Lepr. 36 (1968) 288-295.
4. COURTRIGHT, P. D. Defining the magnitude of ocular complications From leprosy: problems of methodology. Int. J. Lepr. 56 (1988) 566-573.
5. CRAWFORD, C. L., EVANS, D. H. L. and EVANS, E. M. Experimental neuritis induced by sensory nerve myelin may provide a model for non-Iepromatous leprosy. Nature 251 (1974) 223-225.
6. CRAWIORD, C. L. and HOBBS, M. J. Why is the cervical branch of the facial nerve not enlarged in leprosy? J. Anat. 184(1994) 188.
7. DASTUR, D. K. Pathology and pathogenesis of predilective sites of nerve damage in leprous neuritis. Neurosurg. Rev. 6 (1983) 139-152.
8. DASTUR, D. K., ANTIA, N. H. and DIVEKAR, S. C. The facial nerve in leprosy. 2. Pathology, pathogenesis, electromyography and clinical correlations. Int. J. Lepr. 34(1966) 118-138.
9. DONNER, T. R. and KLINE, D. G. Extracranial spinal accessory nerve injury. Neurosurgery 32 (1993)907-910.
10. FARRAGHER, D. Trophic electrical stimulation for human muscle in chronic traumatic facial paralysis. Clin. Rehab. 1 (1987) 366-370.
11. FARRAGHER, D., KIDD, G. L. and TALI.is, R. Eutrophic electrical stimulation for Bell's palsy. Clin. Rehab. 1 (1987)265-271.
12. FORSTER, A. and PALASTANGA, N. Clayton's Electrotherapy. 8th edn. London: Bailliere Tindall, 1981, pp. 70-76.
13. HOGEWEG, M., UDAYA KIRAN, K. and SUNEETHA, S. The significance of facial patches and type I reaction for the development of facial nerve damage in leprosy; a retrospective study among 1226 paucibacillary leprosy patients. Lepr. Rev. 62 (1991)143-149.
14. LUBBERS, W. J., SCHIPPER, A., HOGEWEG, M. and DE SOLDENHOFF, R. Paralysis of facial muscles in leprosy patients with lagophthalmos. Int. J. Lepr. 62(1994) 220-224.
15. LUBBERS, W. J., SCHIPPER, A., HOGEWEG, M. and DE SOLDENHOFF, R. Eye disease in newly diagnosed leprosy patients in eastern Nepal, l.epr. Rev. 65(1994) 231-238.
16. MATSUNE, S., SHIMA, T., OHYAMA, M., SAKAMOTO, K. and TSURUMARU, H. Immunohistochemical study on temporal bone of autopsy eases with Mycobacterium leprae infection. Nippon Jibiinokoka Gakkai Kaiho 98 (1995) 1881-1886.
17. MIKO, T. L., LEMAITRE, C, and KINFU, Y. Damage and regeneration of peripheral nerves in advanced treated leprosy. Lancet 342 (1993) 521-525.
18. RANNEY, D. A. The prevalence and consequences of mis-rcinnervation in facial neuritis. Int. J. Lepr. 42(1974) 316-322.
19. REICHART, P. A., SRISUWAN, S. and METAH, D. Lesions of the facial and trigeminal nerve in leprosy-an evaluation of 43 cases. Int. J. Oral Surg. 11 (1982) 14-20.
20. SABIN, T. D., HACKETT, E. R. and BRAND, P. W. Temperatures along the course of certain nerves often affected in lepromalous leprosy. Int. J. Lepr. 42(1974)38-42.
21. SABIN, T. D. and SWIFT, T. R. Diseases of the Peripheral Nervous System. 2nd edn. Philadelphia: W. B. Saunders Co., 1994, pp. 1955-1987.
22. SCHIPPER, A., LUBBERS, W. J., HOGEWEG, M. and DE SOLDENHOFF, R. Disabilities of hands, feet and eyes in newly diagnosed leprosy patients in eastern Nepal. Lepr. Rev. 65 (1994) 239-247.
23. SCOLLARD, D. M., LATHROP, G. W. and TRUMAN, R. W. Infection of distal peripheral nerves by M. leprae in infected armadillos; an experimental model of nerve involvement in leprosy. Int. J. Lepr. 64(1996) 146-151.
24. SHETTY, V. P., ANTIA, N. H. and JACOBS, J. M. The pathology of early leprous neuropathy. J. Neurol. Sci. 88(1988) 115-131.
25. SHETTY, V. P., MISTRY. N. F. and ANTIA, N. H. Serum demyelinating factors and adjuvant like activity of M. leprae; possible causes of early nerve damage in leprosy. Lepr. Rev. 57 (1985) 221-227.
26. TURKOF, E.. TAMBWEKAR, S., MANSUKHANI, K., MlLLESI, H. and MAYR, N. Intraoperative spinal root stimulation to detect most proximal site of leprous ulnar neuritis. Lancet 343 (1994) 1604-1605.
27. TURKOF, E., TAMBWEKAR, S., MANSUKHANI, K., MlLLESI, H. and MAYR, N. Intraoperative electroneurodiagnostics to detect a second granuloma in the cubital area of the median nerves affected by leprosy: a new approach to prevent incomplete surgery. Int. J. Lepr. 63 (1995) 409-416.
28. UDAYA KIRAN, K., HOGEWEG, M. and SUNEETHA, S. Treatment of recent facial nerve damage with lagophthalmos, using a semistandard steroid regimen. Lepr. Rev. 62 (1991) 150-154.
29. WILLIAM, P. L. Gray's Anatomy. 38th edn. Edinburgh: Churchill Livingstone, 1995, pp. 1243-1248.
1. F.R.C.S., Green Pastures Hospital, Pokhara, Nepal.
2. B.A., B.M., B.Ch., SHO Anesthetics, Royal Liverpool University Hospital, Liverpool, U.K.
Reprint requests to B. Richard, Reconstructive Surgeon, Western Regional Hospital, c/o International Nepal Fellowship, P. O. Box 5, Pokhara, Nepal.
Received for publication on 23 August 1996.
Accepted for publication in revised form on 13 January 1997.