- Volume 65 , Number 2
- Page: 178–89
Humoral and cellular immune reactivity to recombinant M. leprae antigens in HLA-typed leprosy patients and healthy controls
A detailed knowledge of the Mycobacterium leprae proteins that are recognized by the cellular and humoral components of the immune system as well as the inter-individual variation of this recognition are necessary for the rational development of diagnostic tests and immunoprophylactic (vaccines) or immunotherapeutic strategies in leprosy.
In order to obtain large amounts of molecularly well-defined proteins of M. leprae, a lambda-gtll expression library was constructed several years ago (28). This library consists of a large number of lambda bacteriophages, each possessing a molecularly engineered recombinant gene comprised of one of many thousand possible M. leprae genes fused to the Escherichia coli betagalactosidase gene. Thus, each bacteriophage produces a recombinant protein consisting of beta-galactosidase linked to a M. leprae gene product. The library of bacteriophages was then screened for the capacity to produce recombinant proteins capable of reacting with serum antibodies From leprosy patients but not control individuals. Using this approach, several laboratories have identified M. leprae genes which encode immunoreactive molecules. Sathish, et al. have performed extensive molecular characterization of more than 45 genes and based on molecular hybridization studies have grouped these genes into eight different complementation groups, the members of each group exhibiting structural similarity (16). Although these genes were originally identified by their reactivity with patients' sera, studies with T-cell clones have indicated that many of them encode antigens which can be recognized by T cells as well (27).
A systematic evaluation of the reactivity of these recombinant antigens with sera and peripheral blood mononuclear cells (PBMC) From a large number of leprosy patients and healthy controls might enable us to determine the degree of correlation between Band T-cell reactivity to a given antigen and the patient's clinical status. In addition, it is well established that among the many factors which contribute to the immune response to an infectious agent, the HLA phenotype of an individual plays a role in directing the T-cell response to a given antigenic peptide. Moreover, several studies have suggested a correlation between HLA phenotypes and certain clinical forms of leprosy (3), Therefore, HLA typing should also be performed on the PBMC of the patients and healthy controls to determine if any correlation exists between response to a given antigen, leprosy and HLA phenotype.
Recently we studied, in collaboration with the Armauer Hansen Research Institute (AHRI) in Ethiopia, humoral and cellular reactivity to several unpurified recombinant M. leprae antigens in leprosy patients and controls. All antigens were recognized by both antibodies and T cells, and although inter-individual quantitative differences were noted, the data did not indicate a role lor a particular antigen in the induction of or protection From disease. A number of patients who did not respond to the M. leprae sonicate displayed T-cell responses to individual recombinant antigens, in particular the 30/31-kDa (10L) antigen (21).
Because some of the responses in that study may have been influenced by E. coli components, we have used purified recombinant M. leprae antigens in the present study. These purified preparations may yield more and clearer T- and B-cell responses and, therefore, be more useful in analyzing whether correlations exist among antibody reactivity, T-cell reactivity, leprosy status and HLA type.
In order to obtain more information on qualitative differences in immune reactivity, we have also determined the immunoglobulin G subclass responses of individuals who produced antibodies to individual recombinant antigens. Immunoglobulin subclass responses to antigens are differentially regulated by cytokines secreted by different T-helper-cell subsets. Differential T-cell activation has been implicated in leprosy, tuberculoid leprosy being associated with the TH1 subset and lepromatous leprosy with suppressor-T cells and TH2 (11,15).
MATERIALS AND METHODS
Patients and contacts
The PBMC and sera used in this study were collected From patients of the Dr. Sardjito Hospital in Yogjakarta, Indonesia. The study was extended with patients' sera which were originally collected at the Leonard Wood Memorial Center for Leprosy Research, Cebu, The Philippines. The patients were diagnosed according to clinical, bacteriological and histopathological findings and classified according to the criteria of Ridley and Jopling (12). The bacterial index (BI) of each patient was calculated by counting the acid-fast bacteria in slit-skin smears taken From an earlobe or a skin lesion. In total, 114 patients (23 TT, 41 BT, 1 BB, 31 BL, 18 LL), 68 healthy contacts (HC), and 51 healthy blood donors (BD) were included in the study. Twentyfour patients had not started multidrug therapy (MDT), 32 patients had finished MDT, 16 patients were still under treatment, 13 patients did not complete their treatment for unknown reasons, and treatment data of 29 patients were unknown. Patients diagnosed as tuberculoid and having a BI = 0 were defined as paucibacillary (PB) and received 6 months of MDT. Patients with a BI of >0 were defined as multibacillary (MB) and received at least 2 years of MDT or until skin-smear negativity.
From this same group 55 leprosy patients and 49 healthy contacts were tested for their T-cell proliferative responses against recombinant antigens. The patients were grouped as PB (N = 29; 10 TT, 19 BT) and as MB (N = 26; 20 BL, 6 LL). HLA-DRB1 and DQB1 typing was performed on 48 healthy contacts and 57 leprosy patients (33 BT/TT and 24 BL/LL). The healthy contacts were not genetically related to any of the patients involved in this study.
Sample collection
Blood was collected by venipuncture using heparinized vacutainer tubes (Becton and Dickinson, Mechelen, Belgium). PBMC were isolated by Ficoll-Isopaque and then centrifuged. The pelleted cells were resuspended in culture medium (Iscove's modified Dulbecco's medium) supplemented with penicillin (100 IU/ml; GIBCO Laboratories, Grand Island, New York, U.S.A.) and glutamine (2mM; GIBCO). Serum was separated From whole blood and stored, without preservative, at -70ºC until use.
Antigens
The antigens used in the enzyme-linked immunosorbent assay (ELISA) and the lymphocyte stimulation test (LST) are listed in Table 1. M. leprae sonicate was obtained From R. Rees (Mill Hill, London, U.K.), and M. tuberculosis sonicates From A. Kolk (KIT, Amsterdam, The Netherlands).
Purified recombinant proteins M. leprae heat-shock protein (hsp)2L-l, hsp2L-2 and 4L were obtained From J. van Embden through the WHO/TDR/IMMLEP Special Programme. The M. leprae recombinant proteins 10L(B), 25L and 43L were overexpressed in the pTrcHIS (Invitrogen Corp., San Diego, California, U.S.A.) expression
vector and purified under denaturing conditions using a nickel affinity column according to the recommendations of the manufacturer (Qiagen Inc., Diagen, Germany). The overexpressed empty pTrcHIS vector was used as a control for the purified histidinetagged recombinant proteins (His-control) in the ELISA and the LST.
D-BSA, a semisynthetic antigen which contains the immunodominant 3,6-di-omethyl-glucopyranosyl moiety of phenolic glycolipid-I (PGL-I) was a kind gift From the World Health Organization (WHO).
Serological assays
The sera were analyzed using ELISAs for the detection of IgG antibodies against the recombinant antigens 10L(B), 25L, 43L and 2L-1 and for the detection of IgM antibodies against PGL-I using a semisynthetic antigen (D-BSA). The ELISAs were essentially performed according to the method of Brett, etal. (1).
The antigens were diluted in carbonate buffer (pH 9.6) and coated overnight at 37ºC in a moist chamber onto wells (50 µl/well) of polystyrene 96-microwell plates (Nunc, Roskilde, Denmark). The concentrations of the antigens were: 0.1 µg/ml D-BSA, lOug/ml 10L(B), 1 µg/ml 25L, 1 µg/ ml 43L and 10 µg/ml 2L-1. The controls used for these antigens were: 0.1 µg/ml BSA for D-BSA and 2L-1, and 10, 1 and 1 µg/ml HIS control for 10L(B), 25L and 43L, respectively. Microtiter plates were blocked for 60 min with 100µl of 1% bovine serum albumin (BSA) in phosphatebuffered saline (PBS) containing 0.05% Tween 20 (PBST). After washing three times with PBST, the sera were diluted 1:300 in PBST containing 10% normal goat serum (NGS) and 50µl was added to each well. When measuring Ig subclasses, sera were diluted 1:50. Serum dilutions were incubated at 37ºC for 60 min; this was followed by another wash step. Peroxidaseconjugated anti-human IgM conjugate (Cappel, West Chester, Pennsylvania, U.S.A.) and IgG conjugate (Cappel) were added (50 µl/well) at a 1:2000 dilution in PBST-10% NGS to microtiter plates coated with D-BSA and plates coated with the recombinant proteins, respectively.
When IgG subclasses were measured, biotin-labeled mouse monoclonal anti-human antibodies specific for each of the IgG subclasses (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) diluted in PBST-10% NGS at concentrations of 1:1000 for IgG 1 (clone 8c/6-39), IgG3 (clone HP-6050) and IgG4 (clone HP-6025) and 1:10,000 for IgG2 (clone HP-6014) were added (50 µl/well). After incubation at 37ºC for 60 min, the washing procedure was repeated and 50 µl of ExtrAvidin peroxidase (Sigma) diluted to 1:1000 in PBST-10% NGS was added in each well.
After incubation with conjugate at 37ºC for 60 min, the washing procedure was repeated and 100 µl of a 0.1 M citratephosphate buffer containing 0.4 mg/ml o-phenylenediamine and 0.0066% hydrogen peroxide was added to each well. In order to control for plate-to-plate and day-today variations, a positive reference serum was included in triplicate on each plate. The color reactions of the entire plate were stopped with 50 µl 2 N H2SO4 when the optical density (OD) From a positive control serum reached an OD value of 0.6. The ODs were measured in a spectrophotometer (Titertek Multiscan) using a 492 nm filter. All sera were tested in duplicate, and the ELISA results were expressed as mean absorbance of the duplicates. The final OD value of each serum sample was calculated by subtracting the OD value of the wells coated with control antigen From the OD values of the test wells coated with D-BSA or one of the recombinant proteins.
Proliferation assay
LSTs were performed as described before 2I) using sonicates of M. leprae and M. tuberculosis in two different concentrations: 2 and 20 µg/ml. Purified recombinant M. leprae antigens were used in three concentrations: 0.2, 2 and 20 µg/ml. Control wells contained either phytohemagglutinin (PHA: 2 µg/ml; Wellcome Diagnostics, Dartford, U.K.) or recombinant IL-2 or the His control or culture medium alone. Cultures were set up in triplicate using 1.5 x 105 cells per well. The results of the responses to sonicates of M. leprae and M. tuberculosis as well as to the purified proteins hsp 2L-1 and 4L are expressed as a stimulation index (SI) which is the ratio of'H-thymidine incorporation of antigen-stimulated cultures to control cultures containing neither antigen nor PHA. The results of the responses to the recombinant proteins 43L, 10L(B) and 25L were expressed as a SI, which is the ratio of 3H-thymidine incorporation of antigenstimulated cultures to cultures containing the His control antigen. The responses against 2L-2, which is a MBP fusion protein, were divided against the response to the overexpressed MBP vector without an insert. A SI of >3 was considered as a positive response. The average PHA/IL2 responses were 14,276/25,018 cpm for the HC (N = 49), 26,312/27,907 for the BT/TT patients (N = 32), and 26,429/27,400 for the BL/LL patients (N = 24).
HLA typing
DNA isolation and "generic" DRB1 typing (medium resolution) and DQB1 typing with the PCR/biotin-SSO method was performed as described (8-24).
Statistical analysis
The results of the lymphocyte proliferation tests were analyzed by chi-squared analysis. For the ELISA the cut-off value for a positive test result for each antigen was taken as the mean OD value of 51 healthy Indonesian blood donors plus two times the standard deviation (S.D.). Analyses of variance methods were done as indicated in the text. All probabilities presented are two-tailed. The study data were analyzed with the statistical program Epi-Info, vs 5.0.
RESULTS
B-cell recognition of M. leprae antigens
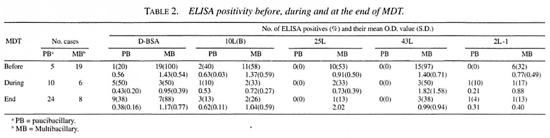
ELISA results of untreated patients. The difference between a positive and a negative test result for each antigen was determined by a cut-off point. The cut-off point values were calculated by taking the mean OD value of 51 healthy Indonesian blood donors plus 2 S.D. The cut-off points for the individual antigens were D-BSA (0.23), 10L(B) (0.52) 42L (0.37), 43L (0.32) and for 2L-1 (0.20). ELISA positivity of sera From 51 blood donors (BD) was 7.8%, 7.8%, 5.9%, 5.9% and 3.9% for the D-BSA, 10L(B), 25L, 43L and 2L-1 antigens, respectively. For the 68 healthy contacts (HC) the seropositivity rates were 5.9%, 4.4%, 8.8%, 0% and 19.1% for the D-BSA, 10L(B), 25L, 43L and 2L-1 antigens, respectively. None of the ELISAs detected significantly more positives in the contact group (HC) compared to the BD group.
The seropositivity rates of the leprosy patients are shown in Table 2. More positivity was found toward the lepromatous pole of the leprosy spectrum. Significantly more untreated MB patients were positive compared to the blood donors for all antigens (p < 0.01 by Fischer exact test). Sensitivity was 100% for LL patients (N = 10) in the D-BSA and 43L ELISAs (results not shown). Seropositivity in untreated PB patients could only be detected using D-BSA and 10L(B) as antigen.
The percentage of seropositivity to the recombinant antigens declined during treatment; this was less clear for the anti-D-BSA antibodies.
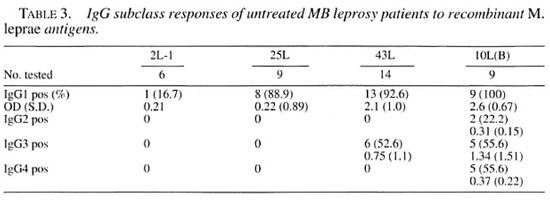
IgG subclass antibody response to different recombinant M. leprae antigens in untreated patients. A number of the sera positive to one of the recombinant antigens were retested for IgG subclass determination. Table 3 shows the difference in reactivity in the IgG subclass antibody responses of untreated patients to the M. leprae 10L(B), 43L, 25L and 2L-1 antigens. Antibody responses were mainly of the IgG1 subclass. IgG 1 was detected against 10L(B), 43L and 25L in more than 80% of the patients but only 1 out of 6 patients had IgG 1 to 2L-I (chi-squared test p = ().()()() 14). The IgG3 response was present in about 50% of the patients against two of the antigens, 10L(B) and 43L. IgG2 and IgG4 antibodies could be detected only against 10L(B). Thus, all IgG subclasses could only be detected to 10L(B).
IgG subclass responses to 10L(B) in six leprosy patients who developed reversal reactions during the course of treatment. The Figure shows the antibody levels during the course of treatment in six patients who developed type 1 reversal reactions. In all patients only IgG 1 was detectable at significant levels. Patient number 1 had a low IgG4 response in month 12 of treatment; no further responses were detected in IgG2, IgG3 or IgG4 in these six patients (data not shown). A diversity in the IgG 1 subclass responses is clearly seen, but no relation could be found with the onset of a reversal reaction.
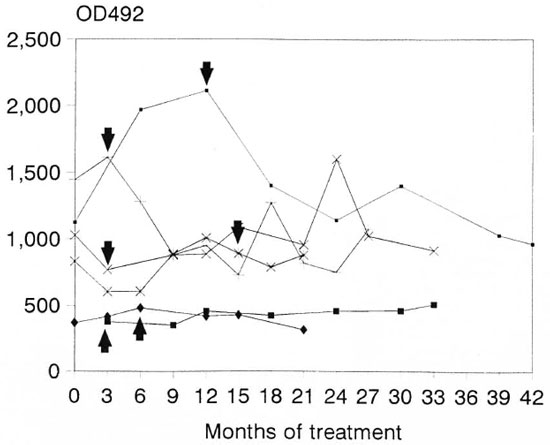
The Figure. IgGl responses to 10L(B) in leprosy patients during the course of treatment. = Patient 1 ; + = patient 2; * = patient 3,
= Patient 1 ; + = patient 2; * = patient 3,  = patient 4; x = patient 5;
= patient 4; x = patient 5;  = patient 6. Arrows indicate occurrence of a reversal reaction.
= patient 6. Arrows indicate occurrence of a reversal reaction.
Proliferative T-cell responses to M. leprae antigens. Pilot experiments demonstrated that freshly isolated human PBMC gave significantly higher T-cell proliferative responses than frozen PBMC upon stimulation with mycobacterial and control antigens (data not shown). Th erefore, we performed our studies on PBMC within 24 hr after collection of blood samples. We tested the six M. leprae recombinant antigens listed in Table 1. Table 4 shows the results of subjects demonstrating positive reactivity of PBMC with the antigens studied as assessed by the proliferation assay. Subjects are grouped according to their disease status (HC, BT/TT and LL/BL). The frequency of responses to whole M. tuberculosis sonicate was high (more than 90% of subjects). In addition, high frequencies of positive responses to whole M. leprae sonicate were found in the BT/TT group (93%) and in the HC group (61%); 35% of the BL/LL patients responded to the M. leprae sonicate. Of the antigens tested, 4L and 43L were the most frequently recognized antigens (between 18%-25% in all three groups). In this study only a small percentage of individuals responded to 2L-1(0-10%), 10L(B) (10%-12%) and to 25L(3%-8%). 2L-2 was recognized by one patient, and none of the hsp2L-1 responders recognized this protein, which shows 59% homology with 2L-1. None of the antigens was recognized by only one particular sub-ject group, which argues against a particular role in protection or disease. We found no differences in T-cell responses to any M. leprae antigen between MDT treated and untreated patients.
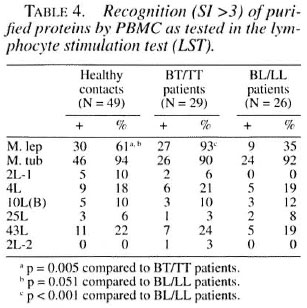
Comparison of B-cell versus T-cell responses. The antigens 2L-1, 10L(B), 25L and 43L were tested for recognition by both T and B cells. The results are subdivided for healthy contacts, BT/TT patients and BL/LL patients and shown in Table 5. Antibody and/or T-cell reactivity to these four recombinant M. leprae antigens was observed in only 3% (25L in BT/TT patients) to 46% (43L in BL/LL patients) of the individuals tested. In all groups the 43L antigen was most often recognized. There was a clear inverse correlation between the LST and ELISA positivity for all antigens tested and in all three groups of individuals.
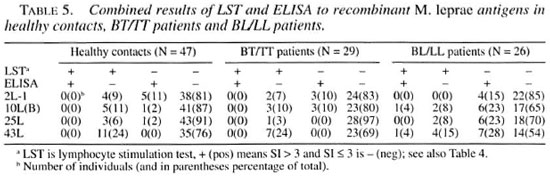
HLA and disease status. Significant associations (before correction for the number of comparisons made) between the HLA-DRB1 and -DQB1 alleles and leprosy or leprosy type are shown in Table 6. An association was observed between HLA-DRB 1 *02/* 15 and both subgroups (tuberculoid and lepromatous) of leprosy, confirming a previous study in the same population (Soebono, etal. I8). In that study we also observed a negative association between DRB1*12 and leprosy, which was not found in the present study and, therefore, probably has been due to chance. The same probably applies to the negative association between DRB1*07 and leprosy which was observed in the present but not in the previous study. This study is the first in this population where DQB 1 typing was performed and reported. Therefore, the association between DQB 1*0601 and lepromatous leprosy is of interest.
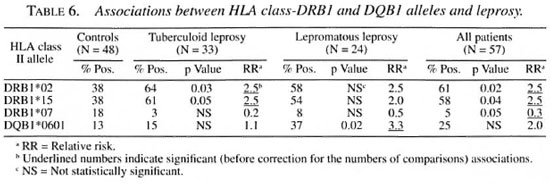
The numbers of HLA-typed individuals positive in the ELISA and/or LST was too small to allow a meaningful analysis of the relationship between antibody and T-cell reactivity to M. leprae antigens and HLA type.
DISCUSSION
In this study the humoral immune responses to five recently described recombinant M. leprae antigens in leprosy patients, healthy contacts and blood donors were evaluated. In general, seropositivity rates were higher for MB patients than for PB patients, and declined with treatment. This is in accordance with the results which have been reported before in immunoblotting studies using the 10L(B) (22), 25L and 43L (14,26) and in ELISA using D-BSA (1), Either IgGl or IgGl and IgG3 subclass responses were detected in most of the MB patients to some or all of the recombinant antigens. The level of IgG3 generally was lower than IgGl, which is comparable to their proportion in whole serum. A higher level of IgG 1 and IgG3 subclasses has been found in other studies using recombinant M. leprae 18-kDa antigen (6) and M. leprae sonicate (7).
Although IgG 1 and/or IgG3 clearly were the predominant subclasses against all recombinant antigens tested, there was also evidence for antigen-dependent subclass diversity: all IgG subclasses could be detected against 10L(B), IgGl and IgG3 against 43L, while only IgGl could be detected against 25L and 2L-1. In conformity with our results, only IgGl was found in another study using 2L-1 (l7). Both the 10L(B) and 43L antigens to which we detected other than IgGl subclass responses are secreted proteins of M. leprae (4-23). This is in favor of the idea that location and mode of antigen expression may have an effect on the type of immune response induced by mycobacteria (19).
Production of IgGl and IgG3 subclasses is believed to be associated with the TH1 subset of T-helper cells, through upregulation by gamma-interferon (IFN-γ) produced by the activated T cells (5). The appearance of these subclasses in lepromatous leprosy patients in whom TH1 responses are, in general, low or absent, has therefore been considered as surprising (7). One explanation may be that alternative sources of IFN-γ exist. Indeed, IFN-γ has been found to be secreted by natural killer (NK) cells (25) and also by gamma-delta T cells (2). On the other hand, IL-2 and IL-4 also have been implicated in augmentation of IgGl and IgG3, respectively (9).
In treated patients the levels of IgG subclass decreased. This may be due to an increased binding of antibodies to antigens liberated when the bacteria are killed, which would decrease the serum level since antibody participates in local elimination of the dead bacteria. There may also be a down-regulation of antibody production as a result of the effects of drug therapy on the dynamics of the infection and immune response to it, or even a combination of both.
In none of the cases was there evidence for an immunoglobulin subclass switch during or preceding a reactional state, nor for a selective decrease of one subclass of antibody. The interval between serum collection and onset of the reactional state may have been too long. On the other hand, we cannot exclude the possibility that the de tection limit of our assays was too low and that a class switch may be found when us ing lower dilutions of serum.
T-cell reactivity against the mycobacte rial sonicates and purified recombinant anti gens was tested in a lymphocyte stimulation test (LST). The main purpose of the present study was to test whether purified recombi nant antigens would yield more specific T-cell responses as compared to the unpuri fied preparations used in a recent study in Ethiopia (21). Therefore, we will specifically compare the results of the present study with that study. A majority (>90%) of the individuals displayed T-cell reactivity against the M. tuberculosis sonicate, irre spective of the leprosy status. Similar re sults were obtained in Ethiopia (21) and this reactivity has to be kept in mind when in terpreting the results of the T-cell reactivity against the recombinant antigens. Compared to our Ethiopian study, T-cell reactiv ity against M. leprae sonicate was lower in healthy contacts (61% versus 95%) and higher in both tuberculoid leprosy (93% versus 72%) and lepromatous leprosy pa tients (35% versus 11%). Four of the puri
1. Ph.D.; Department of Biomedical Research, Royal Tropical Institute, Amsterdam, The Netherlands.
2. M.Sc, Department of Biomedical Research, Royal Tropical Institute, Amsterdam, The Netherlands.
3. Professor, Department of Immunohematology and Bloodbank, Universitv Hospital, Building 1, E3-Q, P. O. Box 9600, 2300 RC Leiden, The Netherlands.
4. Ph.D.; Professor, Department of Immunohematology and Bloodbank, Universitv Hospital, Building 1, E3-Q, P. O. Box 9600, 2300 RC Leiden, The Netherlands.
5. Professor, Department of Immunohematology and Bloodbank, Universitv Hospital, Building 1, E3-Q, P. O. Box 9600, 2300 RC Leiden, The Netherlands.
6. M.D., Professor, Department of Immunohematology and Bloodbank, Universitv Hospital, Building 1, E3-Q, P. O. Box 9600, 2300 RC Leiden, The Netherlands.
7. H. Soebono, M.D., Department of Dermatology, Gadjah Mada University, Yogyakarta, Indonesia.
Reprint requests to Prof. Dr. R. R. P. de Vries at the address above or FAX 31-71 -521 -6751.
Current address for Dr. Thole; Department of Medical Microbiology, Imperial School of Medicine at St. Mary's, London, U.K.
Received for publication on 29 May 1996.
Accepted for publication in revised form on 3 March 1997.