- Volume 65 , Number 2
- Page: 203–10
Association between leprosy and HIV infection in Tanzania
ABSTRACT
Setting: An epidemiological study of the interaction of leprosy and HIV infection in Tanzania. Objective: To establish the prevalence of HIV infection among leprosy patients, and to measure the association of HIV and leprosy by comparing the HIV prevalence in leprosy patients and blood donors. Design: Testing for HIV infection in consecutively diagnosed leprosy patients (new and relapsed after MDT) in all regions in Tanzania successively for a period of 3 to 6 months during 1991, 1992 and 1993. Results: Out of the total estimated eligible leprosy patients, 697 patients (69%) entered the final analysis. The HIV prevalence among these leprosy patients was 12% (83/697) as compared to 6% (8960/158,971) in blood donors examined in Tanzania during the same period. There were no significant differences in HIV seroprevalence by age, sex, residence or type of disease. However, the adjusted odds ratio (OR) of the presence of a BCG scar was 1.9 [95% confidence interval (CI) 1.1-3.3] among HIV-positive leprosy cases compared to HIV-ncgative leprosy cases. Comparing leprosy cases with blood donors as controls, the logistic regression model, controlling for sex, age group and residence, showed the OR for HIV seropositivity among leprosy patients to be 2.5 (95% CI 2.0-3.2). This association existed in all strata, but was strongest in the 15-34-year age group. No difference of HIV status between multibacillary and paucibacillary leprosy could be shown to exist. The point estimate of the population attributable risk of HIV infection for leprosy was 7%. Conclusion: HIV infection is associated with leprosy and might reverse the epidemiological trend of the slow decline in case notification in Tanzania if HIV infection is increasing greatly. Previous BCG vaccination loses its protection against leprosy in the presence of HIV infection. A repeated study is recommended in order to validate these findings, whereby recording of the disability grading of the cases is necessary to adjust for delay in diagnosis.RÉSUMÉ
Cadre: Une étude épidémiologique de l'interaction entre la lèpre et l'infection VIH en Tanzanie. Objectif: Etablir la prévalence de l'infection VIH chez les malades de la lèpre, et mesurer l'association entre le VIH et la lèpre en comparant la prévalence VIH chez des malades de la lèpre et des donneurs de sang. Méthode: Tester pour l'infection VIH chez des malades de la lèpre diagnostiqués successivement (nouveaux et rechutes après PCT) dans toutes les régions de Tanzanie, l'une après l'autre pendant une période de 3 à 6 mois au cours des années 1991, 1992 et 1993. Résultats: Du total des patients lépreux estimés éligibles, 697 malades (69%) ont fait partie de l'analyse finale. La prévalence VIH par mi ces malades de la lèpre était de 12% (83/697), en comparaison aux 6% (8960/158971 ) chez les donneurs de sang examinés en Tanzanie durant la même période. Il n'y avait pas de différence significative dans la séroprévalence du VIH par âge, sexe, la résidence ou le type de la maladie. Cependant, l'odds ratio ajusté (OR) de la présence d'une cicatrice de BCG était de 1.9 [limites de confiance (LC) à 95% 1.1-3.3) parmi les malades de la lèpre positifs pour le VIH, comparé aux cas de lèpre négatifs pour le VIH. En comparant les cas de lèpre avec les donneurs de sang comme témoins, le modèle de régression logistique, contrôlant pour le sexe, le groupe d'âge et la résidence, a montré que l'OR pour la séropositivité au VIH parmi les malades de la lèpre était de 2.5 (LC 95% 2.0-3.2). Cette association existait dans toutes les strates, mais était la plus forte dans le groupe d'âge 15-34 ans. On n'a pas pu montrer qu'il existait une différence de statut VIH entre la lèpre multibacillaire et la lèpre paucibacillaire. L'estimation ponctuelle du risque attribuable de l'infection VIH pour la lèpre était de 7% . Conclusion: L'infection VIH est associée à la lèpre et pourrait inverser la tendance épidémiologique de diminution lente dans la notification des cas en Tanzanie si l'infection à VIH augmente fortement. Une vaccination antérieure par le BCG perd son pouvoir protecteur en présence d'une infection à VIH. La répétition de cette étude est recommandée pour valider ces observations, et l'enregistrement du taux d'incapacité des cas est nécessaire pour ajuster pour le délai au diagnostic.RESUMEN
Se trata de un estudio epidemiológico sobre la asociación entre la lepra y la infección por el VIH en Tanzania. El objetivo fue el establecer la prevalência de la infección por el VIH entre los pacientes con lepra y la medición del grado de asociación entre las dos enfermedades, por comparación de las prevalências de VIH en los pacientes con lepra y en donadores de sangre sanos. En el estudio se buscó la infección por VIH en los pacientes con lepra de Tanzania (Nuevos y recayentes después de la poliquimioterapia) por periodos consecutivos de 3 a 6 meses durante los años 1991, 1992 y 1993. Sólo 697 del total de los pacientes elegibles (69%) entraron en el análisis final. La prevalência de VIH en los pacientes con lepra fue del 12% (83/697), en comparación con el 6% (8960/158,971) encontrado en los donadores de sangre sanos. No hubieron diferencias significativas en la prevalência de HIV en relación a la edad, sexo, residência, o tipo de Ia enfermedad. Sin embargo. Ia probabilidad ajustada, tomando en cuenta Ia presencia de una cicatríz por BCG, lue de 1.9 (95% de intervalo de confianza (1C) 1.1.-3.3] en los pacientes con lepra VIII positivos, en comparación con los pacientes VIII negativos. Por otro lado, la comparación de los casos de lepra con los indivíduos sanos utilizando un modelo de regresión logístico controlado en cuanto a sexo, edad y residência, mostro que la probabilidad de seroposítividad para VIII entre los pacientes con lepra fue de 2.5 (95% Cl 2.0-3.2). Esta asociación existió en todos los estratos pero fue más marcada en el grupo de edad entre 15 y 34 anos. No se encontro diferencia dei estatua VIH entre los pacientes multibacilares y paucíbacilares. El riesgo estimado de Ia población a Ia infección por VIII fue dei 7%. Se concluye que Ia infección por VIH está asociada con Ia lepra y que el incremento de Ia infección por VIH podría revenir la tendência epidemiológica de la baja notification de casos de lepra en Tanzânia. La vacunación previa con BCG deja de proteger contra le lepra cuando ocurre Ia infección por VIH. Se recomienda repetir el estúdio para validar estos hallazgos, tomando en cuenta Ias incapacidades de los pacientes y el grado de las mismas a fin de corregir los posibles retardos en el diagnóstico.The relationship between leprosy and infection with the human immunodeficiency virus (HIV) and its impact on the epidemiology of leprosy remains controversial (9). Some population-based (2) and hospitalbased (8) epidemiological studies have shown an association between the two, as has one study in an animal model (5). Other hospital- and population-based studies could not establish such an association (8,l0,13,15-l7),and one study suggested a negative association between leprosy and HIV infection (7). An earlier report From Tanzania (3) on the prevalence of HIV infection among leprosy patients using blood donors and antenatal women as controls did not demonstrate an association. HIV infection has had a significant impact on the epidemiology of tuberculosis as evidenced by numerous reports, including several From Tanzania (4-l8), where case notifications of tuberculosis have been increasing dramatically in large part as a result of the HIV epidemic. HIV infection has a well-established relationship with mycobacterial diseases in general, and one might expect that HIV infection is also associated with leprosy.
In contrast with tuberculosis, case notifications of leprosy in Tanzania have not changed appreciably over the past 13 years (The Figure). Total case notifications decreased by 79%, From 3208 to 2537 cases, between 1983 and 1995. However, the case notifications of multibacillary (MB) patients showed an increase of 33%, From 660 to 876 cases, during the same period.
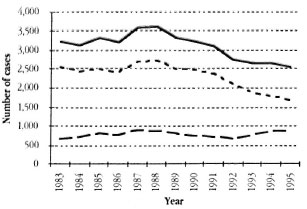
The Figure. Trend in leprosy case notification (new cases and relapses after MDT) in Tanzania, 1983-1995. - - - = Paucibacillary; - - - multibacillary; - = total.
The proportion of MB cases among the new cases detected, which is expected to increase when the risk of leprosy infection decreases and the cases with the longer incubation period become more prevalent, changed little except for the last 3 years. The proportion of notifications of relapses following multiple drug therapy (MDT) remained constant over the past 13 years, averaging <5% of the newly notified cases.
In this paper we present the results of a study of HIV infection among all new and relapsed leprosy patients notified between 1991 and 1993 throughout Tanzania.
MATERIALS AND METHODS
Leprosy cases enrolled in the study were consecutively diagnosed, new and relapsed self-reported MB and paucibacillary (PB) leprosy patients in all 20 regions of Tanzania. The period of enrollment varied From 3 to 6 months per region and all regions were covered in the 3-year period 1991-1993. The general plan of the study has previously been reported (4).
The case definitions used were: a) MB leprosy patient: a leprosy patient with clinical signs compatible with borderline lepromatous (BL) or lepromatous (LL) leprosy, or a patient with a positive bacterial index (BI) of the skin smears examined in the local laboratory; and b) PB leprosy patient: a leprosy patient with clinical signs compatible with tuberculoid (TT) or borderline tuberculoid (BT) leprosy and with a negative BI of the skin smears examined in the local laboratory.
HIV sero-status was based on the results of a single ELISA (Vironostika anti-HTLV-III, Organon Teknika, The Netherlands) performed on a sample of venous blood taken immediately after diagnosis.
The controls were blood donors screened for HIV in the regions of Tanzania between 1991 and 1993 by the National AIDS Control Programme, stratified by sex, age groupand urban or rural residence. This HIV screening used the result of a single HIV check in district hospitals and the result of asingle [LISA at regional hospitals.
Multivariate analysis was done by logistic regression and the Mantel Haenszel analysis in order to calculate adjusted odds ratios. Cases and controls were stratified according to HIV status, sex, age group and residence. Statistical analysis was done with Stata and Microsoft Excel computer software.
RESULTS
A total of 731 leprosy patients were enrolled in the study and tested for HIV infection. These patients represented 69% of the notified new and relapsed cases reported in the various regions during the study periods. A summary of the intake of leprosy patients by year and per region with the percentage of eligible leprosy patients testing positive for HIV infection is presented in Table 1. For the final analysis patients under 15 years of age, those with a classification other than new or relapsed, and those patients with missing information (i.e., disease classification, age, gender or HIV status) were excluded. The characteristics of the 679 new and relapsed leprosy patients with complete data are shown in Table 2.
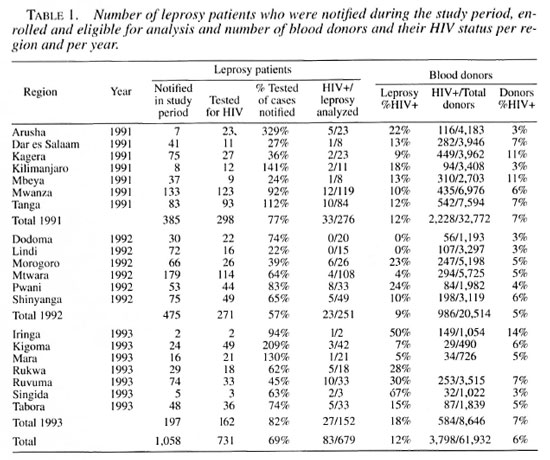
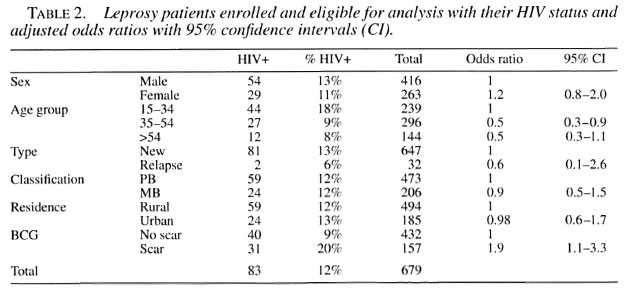
The overall HIV prevalence in leprosy patients was 12% (83/679). After stratification of the leprosy cases no statistically significant difference in HIV status could be shown to exist between sex, type of disease (new or relapsed after MDT), classification (PB or MB) or residence (urban or rural districts). Patients in the 35-54-year age group were significantly less likely to have HIV infection compared to patients between 15 and 34 years of age.
Patients with a BCG scar were nearly twice as likely to have HIV infection compared to patients without a BCG scar, after controlling for age, sex, type of disease, classification and residence.
The HIV prevalence among the total cumulative number of blood donors who were tested during 1991, 1992 and 1993 in Tanzania was 6% (8960/158,971). Using blood donors as controls, the overall odds ratio for HIV infection among leprosy patients calculated From a logistic regression model and controlling for sex, age and residence was 2.5 (95% confidence interval 2.0-3.2). A Mantel Haenszel analysis, stratified for sex, age group and residence, indicated that this association existed in all strata, with the strongest association in the 15-34-year age group. Point estimates of the attributable risk and the population attributable risk was 57% and 7%, respectively. The results of these calculations are presented in Table 3.
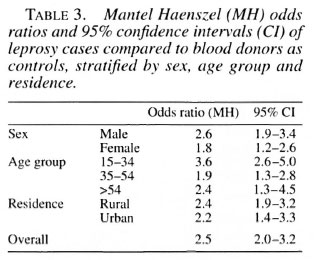
Separate analyses were not performed for MB or PB cases because no significant difference could be shown to exist between the two classifications.
DISCUSSION
This study represents the largest assessment of the impact of HIV infection on leprosy yet undertaken. The results suggest that HIV infection significantly increases the risk of leprosy, although in Tanzania HIV infection appears to account for relatively few additional cases compared with those which arc not HIV associated. However, there are several possible sources of bias which should be considered when interpreting the results of this study.
Sampling bias, both by under- and over reporting of new cases, could have occurred. The enrollment of cases was 69% of the notified, self-reporting cases in the same regions during the study period. The reasons for incomplete intake were mostly logistic, such as late start of the study, transport problems and temporary absence of the District Tuberculosis and Leprosy Coordinator due to illness. In one district the patients detected by a school survey were excluded From the analysis. In some districts over-sampling occured as a result of including leprosy cases diagnosed just before the start of the study period and following its conclusion, explaining the higher enrollment than the corresponding quarterly reports would suggest.
Although self-reporting has been consistently the national case detection policy in Tanzania since 1979, the number of self-reporting leprosy cases is only an approximation of the true incidence of leprosy. The extent of deformity is correlated with the time the patient had undiagnosed and untreated leprosy. In this study, the disability grading was not recorded and, therefore, no comparison could be made between nondisabled and disabled patients. A case ascertainment bias could have occurred in favor of cases with easy access to health services in urban and less remote rural areas. Because HIV transmission is higher in urban areas we, therefore, may have overestimated the true HIV infection in leprosy cases, although in the analysis we have controlled for residence. In addition leprosy patients who are feeling sick because of concomitant conditions including HIV infection are more likely to attend health services than relatively symptom free leprosy patients. Likewise, patients with a higher socioeconomic status are more likely to have had a BCG vaccination. We could, however, not control for socioeconomic status in either cases or controls.
Based on the results of a study of the HI V prevalence among blood donors as compared to the general population conducted in 1991 in the Mwanza region ('), the use of blood donors as controls is valid, if age, sex and residence are controlled. However, it is possible that since 1991 HIV-infected individuals are increasingly avoiding blood donation. The HIV prevalence among all blood donors tested in Tanzania was 5% in 1990, 6% in 1991, 5% in 1992 and 6% in 1993, not showing any positive trend. This bias in estimating the HIV prevalence in blood donors as controls could not be ruled out and, if present, would also overestimate the association between leprosy and HIV infection. We could not rule out the effect of false-positive results of testing for HIV infection by ELISA or Western blot in leprosy as was suggested by one study (6), and this may have caused a higher HIV seroprevalcnce in leprosy patients. However, we found no further evidence of this effect in any of the other published epidemiological studies of leprosy. We could not rule out the effect of bias resulting From the different HIV testing strategies between leprosy cases (ELISA) and blood donors (HIV check), respectively.
In considering the possible relationship between HIV and leprosy, one must question which of the two infections occurred first. Both HIV and leprosy infection have long incubation periods before breaking down to disease. In addition, leprosy patients tend to wait a long time before seeking medical advice, often only when deformities and disabilities are already established. During this long incubation period, a leprosy patient would be at equal risk to contract HIV infection as a normal individual, unless leprosy would be associated with higher risk sexual behavior than average. This is an unknown factor, although in some reports an association between sexually transmitted disease, but not HIV, and leprosy has been shown (12).
On theoretical grounds it is plausible (11) that the interaction of leprosy and HIV infection would eventually lead to an increase in leprosy cases because of uninhibited multiplication of Mycobacterium leprae in patients with HIV-impaired immunity. This might result in an atypical presentation of MB leprosy cases which would remain as an unrecognized source of infection in the community, thus increasing the risk of leprosy infection. On the other hand, increased mortality related to HIV infection during the long incubation period of leprosy might limit the period of infectiousness. This effect would be greatest in leprosy patients with the longest inculcation period and, therefore, could lead to a relative increase in the notification of (HIV-positive) PB cases.
In Tanzania we have seen no increase in the proportion of PB cases, nor in the proportion of MB cases, except during the past 3 years. This slight increase of the proportion of MB cases was probably due to a 1992 change in the case definition of MB leprosy in Tanzania. The new case definition of MB leprosy then became "any leprosy patient with a positive skin smear result or any leprosy patient with 10 or more lesions." In this study no difference between HIV seroprevalence among MB or PB patients could be shown. This lack of differential association with MB or PB disease could be explained as a possible lack of true association.
Over the past 9 years in Tanzania we have not observed an increase in mortality during treatment of PB or MB leprosy patients in the routine cohort reports. The average reported mortality during treatment was 0.8% among PB leprosy patients and 3.0% among MB patients. Especially among the MB cases, who remain under treatment and observation for a period of 3 years, increased mortality could well be noted and would be indicative for increased mortality due to comorbidity with HIV infection.
In this study we found that the odds ratio of having a BCG scar among HIV-positive leprosy patients, adjusted for sex, age, type, classification and residence, was 1.9 (95% confidence interval 1.1-3.3). An explanation for this finding may be that the protection against leprosy of a previous BCG vaccination is lost due to HIV infection. This is a similar finding as observed among tuberculosis patients enrolled in the parallel study (4) although the magnitude of this association found among leprosy patients is larger than among tuberculosis patients, the odds ratio of having a BCG scar being 1.4 for tuberculosis patients. This would be consistent with the finding (14) that BCG is more protective against leprosy than against tuberculosis. However, as mentioned before, we could not rule out the influence of socioeconomic status in relation to BCG vaccination and HIV infection.
CONCLUSION
Based on the results of this study, HIV infection appears to be associated with leprosy in Tanzania. The study data also suggest that the protective effect against leprosy by BCG vaccination is lessened by HIV infection.
However, we could not exclude the possibility that a number of factors may have biased the observed association, notably the fact that cases were self-reporting and that over- and undersampling has occurred. In addition we were unable to adjust for socioeconomic status and realize the limitations of the use of blood donors as controls. The lack of differential association of HIV infection with MB and PB disease points to a weak association. The epidemiological data and the low population attributable risk suggest that the impact of HIV infection on leprosy in Tanzania was relatively small during the study period.
The future implication for the epidemiology of leprosy in Tanzania is that the number of new cases is only likely to increase if the HIV epidemic is accelerating greatly.
This study has entered its second round, and we suggested to include the recording of the disability grading of the leprosy patients in order to be able to adjust for differences in the latent period in the diagnosis of leprosy. In addition we recommended to strictly adhere to the enrollment criteria in order to reach a more complete coverage.
Acknowledgment. We thank all of the Regional and District Tuberculosis and Leprosy Coordinators who assisted in the study, in particular Dr. Swai (Arusha), Dr. Ilmolelian (Dares Salaam), Dr. Singano (Coast), Dr. Machalo (Dodoma), Dr. Ndyamukama (Iringa), Dr. Rwechungura (Kagera), Dr. Mansoer (Kigoma), Dr. bongo (Kilimanjaro), Dr. Abdulrahman (Lindi), the late Dr. Galibona (Mara), Dr. Minja (Mbeya), Dr. Muhombolage (Morogoro), Dr. Njako (Mtwara), Dr. Sowoki (Rukwa), Dr. Mtumbuka (Ruvunia), Dr. van der Steen (Shinyanga), Dr. Kimala (Singida), Dr. Left (Tabora) and Dr. Mohamed (Tanga). We also thank all of the Laboratory Technicians for testing the blood samples for HIV infection for the leprosy cases. We also thank Dr. Swai, Manager of the Tanzania National AIDS Control Programme, for making available data From the blood donor screening. We finally wish to express our gratitude to our secretary, Mrs. C. Malwa, for the data entry. This study was supported by the World Health Organization Global Tuberculosis Programme.
REFERENCES
1. BORGDORFF, M., BARONGO, L., VAN JAARSVELD, E., KLOKKE, A., SENKORO, K., NEWELL, J., NICOLE. A., MOSHA, F., GROSSKURTH, H. and SWAI, R. Sentinel surveillance for HIV-1 infection: how representative are blood donors, outpatients with fever, anemia, or sexually transmitted diseases, and antenatal clinic attenders in Mwanza Region, Tanzania? AIDS 7 (1993) 567-572.
2. BORGDORFF, M. W., VAN DEN BROEK, J., CHUM, H. J., KLOKKE, H., GRAF, P., BARONGO, L. R. and NEWELL, J.N . HIV-1 infection as a risk factor or leprosy; a case-control study in Tanzania. Int. J. Lepr. 61 (1993)556-562.
3. CHUM, H. J., KITUMBA, A. R., GUNZARETH, M. and GRAF, P. Leprosy and HIV in Tanzania. (Abstract) Int. J. Lepr. 61 Suppl. (1993) 65A.
4. CHUM, H. J., O'Brien, R. J., CHONDE, T. M., GRAF, P. and RIEDER, H. L. An epidemiological study of tuberculosis and HIV infection in Tanzania, 1991-1993. AIDS 10 (1996) 299-309.
5. GORMUS, B. HIV infection and leprosy. (Letter) Int. J. Lepr. 62(1994)610-611.
6. KASHALA, O., MARLINK, R., ILUNGA, M., DIESE, M., GORMUS, B., XU, K., MUKEBA, P., KASONGO, K. and ESSEX, M. Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotrophic viruses among leprosy patients and contacts: correlation between HIV-1 crossreactivity and antibodies to lipoarabinomannan. J. Infect. Dis. 169 (1994) 296-304.
7. KAWUMA, H. J., BWIRE, R. and ADATU-ENGWAU, F. Leprosy and infection with human immunodeficiency virus in Uganda; a case-control study. Int. J. Lepr. 62 (1994) 521-526.
8. LEONARD, G., SANGARE, A., VERDIER, M., SASSOU-GUESSEAU, E., PETIT, G., MILAN, J., M'BOUP, S., REY, J L., DUMAS, J. L., HUGON, J., N'GAPORO, I. and DENES, F. Prevalence of HIV infection among patients with leprosy in African countries and Yemen. J. Acquir. Immune Delic. Syndr. 3 (1990) 1109-1113.
9. LUCAS, S. Human immunodeficiency virus and leprosy. Lepr. Rev. 64 (1993) 97-103.
10. MEERAN, K. Prevalence of HIV infection among patients with leprosy and tuberculosis in rural Zambia. BMJ 298 (1989) 364-365.
11. NAAFS, B., CHIN-A-LIEN, R. A. M., TANK, B. and VAN JOOST, T. Human immunodeficiency virus and leprosy. Trop. Geogr. Med. 46 (1994) 119-121.
12. NWOSU, C. M., NWOSU, S. N. and OKOYE, K. C. Immunodeficiency virus and Treponema pallidum infections in Nigerian patients with leprosy. Int. J. STD AIDS 5 (1994) 48-51.
13. OREGE, P. A., FINE, P. E., LUCAS, S. B., OBURA, M., OKEI.O, C, OKUKU, P. and WERE, M. A case control study on human immunodeficiency virus1 (HIV-1) infection as a risk factor for tuberculosis and leprosy in western Kenya. Tuber. Lung Dis. 74(1993) 377-381.
14. PONNIGHAUS, J. M., FINE:, P. E. M., STERNE, J. A. C, WILSON, R. J., MSOSA, E., GRUER, P. J. K., JENKINS, P. A., LUCAS, S. B., LIOMBA, N. G. and BLISS, L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 339(1992)636-639.
15. PONNIGHAUS, J. M., MWANJASI, L. J., FINE, P. E. M., SHAW, M. A., TURNER, A. C, OXHORROW, X. M., LUCAS, B., JENKINS, P. A., STERNE, J. A. and BLISS, L. Is HIV infection a risk factor for leprosy? Int. J. Lepr. 59 (1991) 221-228.
16. SEKAR, B., JAYASHEELA, M., CHATTOPADHYA, D., ANANDAN, D., RATHINAVEL, L., VASANTHI, B., SUBRAMANIAN, M. and RAO, P. S. Prevalence of HIV infection and high-risk characteristics among leprosy patients of south India; a case-control study. Int. J. Lepr. 62 (1994) 527-531.
17. TEKLE-HAIMANOT, R., FromMEL, D., TADESSE, T., VERDIER, M., ABEBE, M. and DENIS, F. A survey of HTLV-1 and HIVs in Ethiopian leprosy paintients. (Letter) AIDS 5 (1991) 108-110.
18. VAN DEN BROEK, J., BORGDORFF, M., PARKER, N., . CHUM, H. J., KLOKKE, A. H., SENKORO, K. P. and NEWELL, J. HIV-1 infection as a risk factor for the development of tuberculosis: a case-control study Tanzania. Int. J. Epidemiol. 22 (1993) 1159-1165.
1. M.D., M.P.H.; H. Dermatologist, Ministry of Health, Tuberculosis and Leprosy Central Unit, Dar es Salaam, Tanzania (Address: Royal Tropical Institute, Wibautstraat 137J, 1097 DN Amsterdam, The Netherlands).
2. M.D., Dermatologist, Ministry of Health, Tuberculosis and Leprosy Central Unit, Dar es Salaam, Tanzania (Address: Royal Tropical Institute, Wibautstraat 137J, 1097 DN Amsterdam, The Netherlands).
3. M.D., M.P.H., Ministry of Health, National AIDS Control Programme, P. O. Box 933, Dar es Salaam, Tanzania.
4. M.D., Tuberculosis Unit, World Health Organization, Geneva, Switzerland (Address: Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Atlanta, Georgia 30333, U.S.A.).
Reprint requests to Dr. J. van den Broek, Van Ommerenstraat 16, 5708 KB Helmond, The Netherlands.
Received for publication on 12 September 1996.
Accepted for publication in revised form on 6 January 1997.