- Volume 71 , Number 3
- Page: 210–7
Viability and drug susceptibility testing of M. leprae using mouse footpad in 37 relapse cases of leprosy
ABSTRACT
Mycobacteria leprae isolates obtained f rom 37 referral relapse cases of leprosy (37 skin and 10 nerve biopsy samples) received during the years 1994-2001, were tested for viability and drug sensitivity in the mouse footpad. A significant M. leprae yield in the footpads of control mice was obtained, with 32/47 (68%) isolates (f rom 26 cases) thus confirming viability. Of the 28 isolates successfully drug tested, 6 (21%) were resistant to one or more drugs. All except one, were multidrug treated cases (5/24 = 21%). One of the isolates was resistant to all three drugs, i.e., dapsone (di-aminodiphenyl sulphone, DDS), rifampin (RFP), and clofazimine (CLF). Two were resistant to two drugs, i.e., DDS and RFP, and each of the others were mono resistant to DDS, RFP, or CLF. Notably, one of the isolates that showed combined resistance to DDS and RFP was derived f rom a borderline tuberculoid case. Also, in one case skin and nerve showed that discordance viz: M. leprae derived f rom skin were resistant to RFP, while those derived f rom nerve tested sensitive to all three drugs, indicating tissue related difference.RÉSUMÉ
Les isolats de Mycobacterium leprae provenant de 37 cas référés de lèpre avec rechute (37 biopsies de peau et 10 biopsies de nerf) reçus entre 1994 et 2001 furent testés pour leur viabilité et leur chimio-sensibilité au moyen du test de la plante des pieds de souris. Une production significative de M. leprae dans les plantes de pied de souris contrôles fut obtenue, avec 32/47 (68%) isolats (de 26 cas), confirmant ainsi leur viabilité. Parmi 28 isolats qui furent testés avec succès pour leur chimio-sensibilité, 6 (21%) furent résistants à un ou plusieurs antibiotiques et tous, sauf un isolat, provenaient de cas traités par la polychimiothérapie (5/24 = 21%). Un de ces isolats était résistant aux trois antibiotiques (DDS, RFP et CLF). Deux étaient résistants à deux antibiotiques (DDS et RFP) et un des isolats restants était mono-résistant à la DDS, la RFP ou la CLF. Il est à noter qu'un des isolats qui a montré une résistance combinée à la DDS et la RFP était dérivé d'un cas tuberculoïde borderline. De plus, chez un cas, les isolats provenant de la peau et du nerf étaient discordants vis-à-vis de leur antibiorésistance : M. leprae dérivées de la peau étaient résistantes à la RFP, tandis que celles dérivées du nerf étaient sensibles aux trois produits, indiquant des différences liées à la localisation tissulaire.RESUMEN
Utilizando el modelo de la almohadilla plantar del ratón, se estudió la viabilidad y la sensibilidad a drogas de los especimenes de Mycobacterium leprae obtenidos de biopsias de 37 casos de lepra recurrente (37 biopsias de piel y 10 biopsias de nervios) durante el periodo de 1994 a 2001. Treinta y dos de los cuarenta y siete especimenes (68%) de 26 casos se reprodujeron significativamente en la almohadilla plantar del ratón, confirmando su viabilidad. De los 28 especimenes que pudieron probarse para drogo-sensibilidad, 6 (21%) fueron resistentes a una o más drogas y de éstos, todos, menos uno, fueron casos multi-tratados (5/24 = 21%). Uno de los especimenes fue resistente a las 3 drogas (DDS, RFP y CLF), dos fueron resistentes a dos drogas (por ejemplo a DDS y RFP), y el resto fueron resistentes a una de las drogas (DDS, RFP o CLF). Notablemente, uno de los especimenes que mostraron resistencia combinada a DDS y RFP fue un caso derivado de un paciente con lepra tuberculoide subpolar (BT). En otro caso, hubo discordancia entre los bacilos de la piel y el nervio, pues mientras que los derivados de la piel fueron resistentes a RFP, los aislados del nervio fueron susceptibles a todas las drogas, indicando una diferencia relacionada con el tejido.Even though over all response to Multi-Drug Therapy (MDT) was very good, a significant increase in the number of cases presenting with recurrent lesions following release from MDT was noted in the foundation's referral clinic. A detailed study was undertaken on these cases, with a view to find the underlying problems. As part of the investigation, Mycobacteria leprae strains isolated from confirmed relapse cases were screened for resistance, wherever possible, to the constituent drugs of the MDT regimen, using the standardized mouse footpad method. This was undertaken on the premise that both primary and secondary resistance to dapsone was rampant when MDT was first introduced (5,13). Moreover, resistance to rifampicin developed more readily when used alone (3). Since the introduction of MDT for leprosy in the year 1982, over 10 million leprosy patients have completed treatment with MDT worldwide (14). It is deemed important to know if there is any indication of drug resistance among the referral cases presenting with relapse in this center. The results thus obtained are presented and discussed in this paper.
MATERIALS AND METHODS
Drug sensitivity profile was studied in a total of 37 proven relapse cases, received during the period 1994-2001. All except 3 cases under investigation were treated with one of the rifampicin-containing multibacillary multidrug treatment (MB-MDT) regimens. Two were dapsone (di-aminodiphenyl sulphone, DDS) mono-therapy cases and one rifampicin (RFP) mono-therapy case.
Definition of Relapse in MDT cases. Patients having completed a course of MDT, remaining symptom-free for a length time, and developing new skin/nerve lesions and/or reactivation of old lesions, or who have become bacteriologically positive.
Two DDS mono-therapy cases which had received 100 mg daily for 20 and 3 years, respectively, turned smear negative. In all the study cases, the type of leprosy and disease activity were further ascertained through clinical as well as histopathological means on relapse, as detailed below.
Biopsy and its processing. Using local anesthesia, a deep incision skin biopsy from a site that showed highest bacterial index (BI) or activity, and/or an involved nerve were biopsied after informed consent. In 27 cases, only a skin lesion was biopsied, and in 10 cases a skin and a nerve lesion each were biopsied. Each biopsy was divided in to two parts. One part was fixed and processed for light microscopy. The second piece, collected in a sterile vial, was processed for bacterial harvesting and used within 24 hours to determint viability and drug susceptibility using the mouse footpad.
Determination of M. leprae load/gm of tissue. Using sterile measures, biopsied tissues weighing around 0.1 to 0.2 gm (in case of nerve it was usually less than 0.1 gm) were minced and homogenized using glass homogenizer and buffered saline. The final volume of the homogenate was maintained at 1 ml per 0.1 gm of tissue. Spot slides were prepared, stained with Carbol fuschsin, and AFB count/ml as well as /gm tissue was determined using the standardized method (15). Tissue suspensions thus obtained were inoculated into the footpads of non-immunosuppressed Swiss/Webster (S/W) mice within 24 hours, to determine the viability and drug sensitivity of M. leprae as follows.
Determination of viability and sensitivity to drugs in the mouse footpad. Random bred S/W female adult mice were used for the study. Both hind footpads were inoculated with 0.03 ml/footpad of homogenate, containing not more than 10,000 bacilli. Each inocula was injected to a maximum of 50 mice, which were further divided into groups of 8 to 10 for testing for sensitivity to a single concentration, each of a) DDS 0.01 gm%, b) RFP 0.03 gm%, c) Clofazimine (CLF) 0.01 gm%. The fourth group was composed of untreated controls. All of the drugs were given to the test mice from day zero (continuous method) (15). Drugs were given through feed, prepared on a day-to-day basis by wet mixing and blending at room temperature. Mice were maintained in an air-conditioned room.
A total of 47 M. leprae isolates derived from 37 cases (in 27 cases only the skin, and in the remaining 10 cases both skin and nerve homogenates) were tested for viability.
The drug susceptibility testing was done in a total of 42 isolates derived from 37 cases (35 skin and 7 nerve homogenates). Susceptibility to all three drugs, i.e., DDS, RFP, and CLF, were tested in a total of 30 cases. For technical reasons, only two drugs, i.e., DDS and RFP, could be tested in 5 cases, only RFP in one case, and only DDS in one case. All except 2, of the M. leprae strains were drug tested in the primary passage from man to mouse. Two of the strains, both derived from borderline tuberculoid cases, were drug tested in the second passage viz. mouse to mouse.
Harvesting of M. leprae from the footpads. In the control untreated group of mice footpads, harvesting for M. leprae was done at 6th, 7th, 8th, and 12th post inoculation months. The drug treated mice were taken for harvest, as a significant fold increase (i.e., 105/footpad M. leprae yield) was noted in the control group of mice. It should be noted that in this counting method, the lower limit of the delectability of acid-fast bacilli = 1 x 104/footpad. A minimum of two per footpad counts were obtained at the first three intervals, and all the remaining mice were harvested at the 12th month. The results of the test were interpreted as (i) sensitive, if only a significant fold increase (1 x 105/footpad) was noted in the control group of mice, (ii) resistant, if a significant fold increase (i.e., 1 x 105/footpad) was noted in any of the drug treated mice, and (iii) inconclusive, if there was no significant fold increase in control mice.
RESULTS
Histopathological findings. In all except 4 cases, the lesion biopsy obtained upon relapse showed features of active borderline-lepromatous to lepromatous leprosy (BL-LL) with tissue BI ranging between 2+ to 6+. In 3 cases, the lesion showed borderline tuberculoid (BT) and one indeterminate (ID) type of pathology.
Viability test results in the control group of mice. A significant M. leprae yield was obtained in the footpads, in a total of 32 isolates (32/47 = 68%) derived from 26 cases (26/37 = 70% of cases). This includes 26 (70%) skin and 6 (60%) nerve biopsy specimens. In the remaining 11 cases (15 isolates) there was no significant M. leprae yield in the footpads. It was also noted that in the 10 cases where both skin and nerve were tested, the viability score of skin and nerve biopsies were comparable in 7 cases. Six showed positive yield with both, one was negative in both, and only skin positivity was seen in 3 cases.
Drug susceptibility test results. Clinical, bacteriological, and the MFP test results (of 12th month harvest only) obtained in 7 of the cases that showed evidence of growth in drug treated mice are given in Tables 1 and 2, respectively. It should be noted that in 6 cases, results were unequivocal. In one case (no. 6), results remain equivocal as the footpad yield was poor in controls in the skin lesion (SL), though the count in the homogenate was very high.
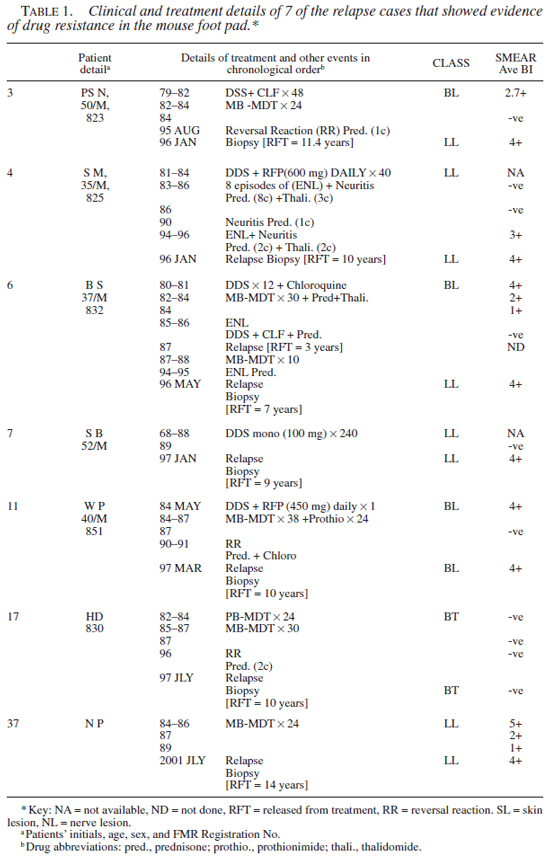
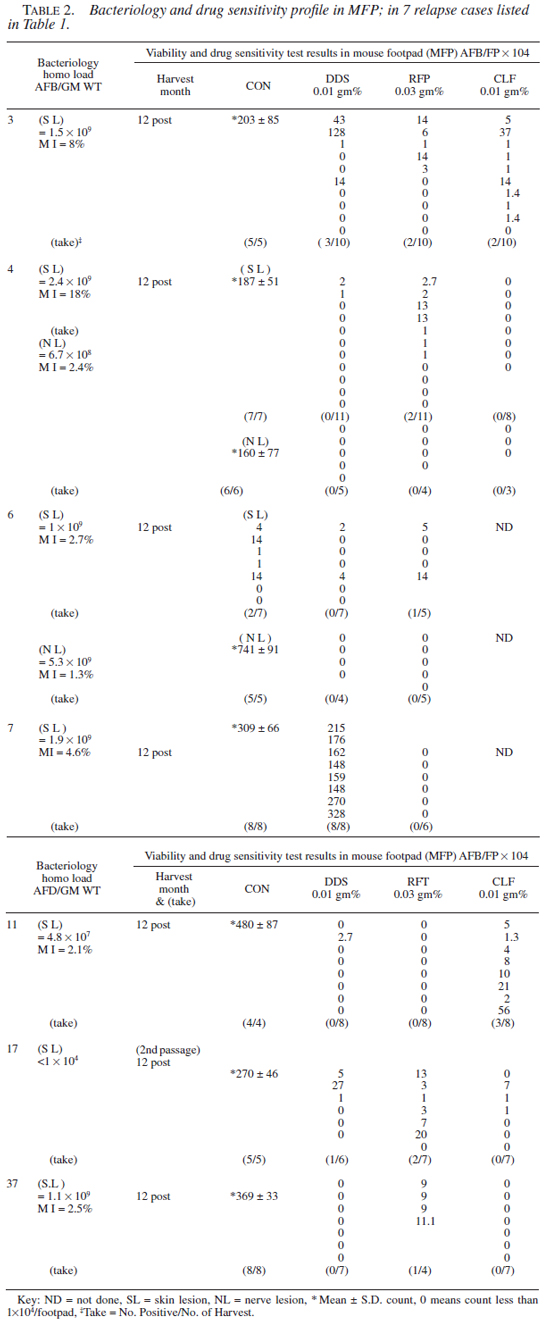
A total of 28 isolates (28/42 from 26 cases) were successfully drug tested (i.e., unequivocal growth of M. leprae in the footpads of control mice). Twenty-two strains (79%) tested sensitive and 6 (21%) showed resistance to one or more drugs. One strain (case 3) was resistant to three drugs, i.e., DDS, RFP, and CLF. Two strains (nos. 4 and 17) were resistant to DDS and RFP, and three were mono-resistant to either RFP, CLF, or DDS (nos. 7, 11, and 37). Significantly, one of the M. leprae strains (no. 17) that showed combined resistance to DDS and RFP was from a smear negative borderline tuberculoid (BT) case. Secondly, the strain that showed a high grade resistance to DDS alone (no. 7) was derived from a case receiving DDS mono-therapy. Thirdly, in one case (no. 4) M. leprae derived from the skin and nerve lesion showed discordance in the drug sensitivity profile. The M. leprae derived from the skin tested resistant to DDS and RFP, while that of the nerve were sensitive to all three drugs. A similar trend was seen with case no. 6, where the results were ambivalent.
Two of the specimens (nos. 3 and 4 both had BI >4+) that tested resistant to RFP in the MFP system were submitted to Dr. S. Cole for the verification of rifampicin resistance using the rpo molecular probe (9). Case no. 3 failed to show rpo mutation, while with organisms from case no. 4, DNA could not be extracted (as per the report by Dr. Cole).
DISCUSSION
Using the mouse footpad method and the standard criteria (i.e., of >1 x 105/footpad yield), we demonstrate that a sizable number of M. leprae isolates (6/28 = 21%) derived from 26 relapse cases (6/26 = 23%) received during the period 1994-2001 harbored M. leprae with acquired resistance to one or more drugs. Five among them (5/26 = 19%) were multidrug treated cases, and the last case showing resistance to RFP had received fixed duration WHO MB-MDT. However in all except one case, footpad yield in the presence of drug did not exceed 25 x 104, whereas in most of the control mice, footpad yield was over 100 x 104. Of the 2 DDS monotherapy relapses included in this study, one showed high-grade resistance to DDS alone. Since only the highest concentrations of drugs were tested, the possibility of resistance to lower concentrations of the drugs, if any, cannot be ruled out.
In the clinical setting, it took a long time to develop the wide spread phenomenon of dapsone resistance. Prevalence of dapsone resistance was as high as 40% to 45% when MDT was first introduced (5,13). Now with 2 decades of MDT usage, it is not very surprising to find M. leprae strains with combined secondary resistance. A case of primary resistance to all three drugs, i.e., DDS, RFP, and CLF was documented by us in the year 1996 using MFP (11). Moreover, there are a few documented cases of resistance to multiple drugs proved using mouse footpad method, and are further ascertained using molecular probes (1,4,7). A retrospective analysis of mouse footpad inoculation results from 1983 to 1997 from the Central Leprosy Training Research Institute (CLTRI), Chingalpettu was carried out recently for the cases with clinical suspicion of relapse/drug resistance (10). Of the 96 cases studied, mono-resistance to dapsone in 15 (16%) and a single case of combined resistance to dapsone and CLF were seen. A majority of the cases (13/16) showed high-grade dapsone resistance, and all were DDS mono-therapy cases. In another similar study from south India, 265 skin biopsies obtained from mixed group of treated and untreated cases of leprosy received between 1987 to1997 were analyzed for primary and secondary resistance using mouse footpad method. In 49 cases, M. leprae strains that were resistant to varying concentrations of DDS, RFP, and CLF were detected (2).
If the results of the present study can be interpreted with the limitations of the protocol used, and testable isolates being only 60% among the multidrug treated cases, occurrence of resistance to RFP was seen in 4/24 (17%), CLF in 2/21 (10%), and DDS in 3/25 (12%). The finding of resistance to RFP and CLF is indeed worrisome, since these are the most important components of current MDT regimen (14).
For reasons not very clear, in one case the drug sensitivity profile between skin and nerve samples showed discordance, in that M. leprae derived from skin was resistant to RFP in tests, while those samples derived from nerve were sensitive to all three drugs. A similar trend was also observed in case no. 6 (Table 2 ). This could be an indication of adaptation of bacteria to differing environments of two tissues (8), and needs to be pursued further. Contrary to expectations, in cases with mono as well as combined drug resistant M. leprae, the cases remained symptom-free for a long time and clinical relapse occurred as late as 9 to 15 years after the cessation of treatment.
Significant questions that remain are why the strain that tested resistant to RFP in the mouse footpad failed to show rpo mutation and secondly, why polymerase chain reaction (PCR) product could not be obtained with the second sample that had >4+ BI. In a recent review article on RFP resistance, it was opined that use of PCR based DNA sequence analysis of the rpo might be a cost-effective alternate technique for diagnosing rifampicin resistance and should supercede the technically difficult MFP (6). However, there are important issues that have yet to be resolved. For example, in a study by Roche and co-workers only 8/60 samples yielded PCR products, and they note that the strain that showed rpo mutation, tested sensitive in the mouse footpad (9).
Time is a major constraint, as the process used for yielding drug susceptibility profiles in MFP takes a long time, 12 months or more. Secondly, even short adverse storage conditions and exposure to anti-leprosy drugs affect the growth of M. leprae in the footpads, resulting in false negativity, as was seen in some of our cases under study. However, it has several advantages that no other tests can offer in the foreseeable future. (i) A strain of interest can be retained and expanded. (ii) A series of drugs can be tested simultaneously. (iii) M. leprae can be isolated from BT lesions and expanded as evidenced in this study and previously (12). And most importantly, (iv) it is a time tested method.
To conclude, keeping the limitations of our study design in mind, the results obtained in MFP suggest resistance to more than one constituent drug of the current MDT regimen, in a small proportion of not only lepromatous but also the tuberculoid leprosy relapse cases.
Acknowledgments. We gratefully acknowledge the help of Dr. N. F. Mistry in the preparation of manuscript, Drs. S. Cole and Nadine Honore for carrying out the rpo PCR test on two of our samples. The financial support for this study was obtained jointly from Leprosy Mission India and Tata Education Trust, Mumbai.
REFERENCES
1. Cambau, E., Perani, E., Guillemin, I., Jamet, P. and Ji, B. Multidrug resistance to dapsone, rifampincin, and ofloxacin in Mycobacterium leprae. Lancet. 349(1997)103-104.
2. Ebenezer, G.J., Norman, G., Joseph, G.A., Daniel, S. and Job, C.K. Drug resistant Mycobacterium leprae-results of mouse footpad studies from a laboratory in south India. Indian J. Lepr. 74(4)(2002)301-312.
3. Grosset, J.H., Guelpa-Lauras, C.C., Bobin, P., Brucker, G., Cartel, J.L., Constant-Desporteus, M., Flageul, B., Frederic, M., Guilllaume, J.C. and Millan, J. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampicin. Int. J. Lepr. Other Mycobact. Dis. 57(1989)607-614.
4. Gupta, U.D. and Katoch, V.M. Drug resistance in leprosy: lessons from past and future perspective-a review. Indian J. Lepr. 71(1999)451-463.
5. Ji, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
6. Ji, B. Rifampicin resistant leprosy: a review and a research proposal of a pilot study. Lepr. Rev. 73(2002)2-8.
7. Matsuoka, M., Kashiwabara, Y. and Namisato, M. A Mycobacterium leprae isolate resistant to dapsone, rifampin, ofloxacin, and sparfloxacin. Int. J. Lepr. Other Mycobact. Dis. 68(2000)452-455.
8. Mekalanos, J.J. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174(1992)1-7.
9. Roche, P.W., Shrestha, N., Thomas, A., Honore, N. and Cole, S.T. Rapid detection of resistance to rifampicin in Mycobacterium leprae. Lepr. Rev. 71 suppl. (2000)S96-S99.
10. Sekar, B., Elangeshwar, N., Jayarama, E., Rajendran, M., Kumar, S.S., Vijayaraghavan, R., Anandan, D. and Arunagiri, K. Drug susceptibility of Mycobacterium leprae: a retrospective analysis of mouse footpad inoculation results from 1983 to 1997. Lepr. Rev. 73(2002)239-244.
11. Shetty, V.P., Uplekar, M.W. and Antia, N.H. Primary resistance to single and multiple drugs in leprosy-a mouse footpad study. Lepr. Rev. 67(1996)280-286.
12. Shetty, V.P., Wakade, A. and Antia, N.H. A high incidence of viable M. leprae in post MDT recurrent lesions in tuberculoid leprosy patients. Lepr. Rev. 72(2001)337-344.
13. WHO Study Group. Chemotherapy of leprosy for control programs. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
14. World Health Organization. Global leprosy situation, September 1999. Weekly Epidemiol. Rec. 74(1999)313-316.
15. World Health Organization. Laboratory techniques for leprosy. WHO/CDS/LEP, 86.4. Geneva: World Health Organization, 1987.
1. M.Sc., Ph.D., Deputy Director, The Foundation for Medical Research;
2. B.Sc., Research Assistant, The Foundation for Medical Research;
3. M.D., D.D.V., Medical Consultant, The Foundation for Medical Research;
4. M.B., B.S., D.D.V., Deputy Director, The Bombay Leprosy Project;
5. B.Sc., M.B.B.S., D.B.V., Director, The Bombay Leprosy Project;
6. FRCS, FACS (Hon.), Director and Trustee, The Foundation for Medical Research, Mumbai.
Reprint Requests to: Dr. V. P. Shetty, The Foundation for Medical Research, 84-A, R.G.Thadani Marg, Worli, Mumbai 400 018, Phone : 91-22-2493 4989 / 2493 2876 (Also Fax), E-mail: frchbom@bom2.vsnl.net.in
Received for publication on 13 May 2003.
Accepted for publication on 8 July 2003.