- Volume 71 , Number 3
- Page: 218–26
Nodular leprosy of childhood and tuberculoid leprosy: a comparative, morphologic, immunopathologic and quantitative study of skin tissue reaction
ABSTRACT
Nodular leprosy of childhood (NL) is a benign clinical variant of tuberculoid leprosy that affects breast-feeding infants and children that remained in a highly infected environment. The lesions resolve with complete healing and NL has been considered a manifestation of allergy and congenital immunity to Mycobacteria leprae. We studied the tissue reaction, Mycobacterial antigen frequency, and the lymphocyte subsets (CD45RO+, CD4+, CD8+, B, NK), dendritic cells (epidermal CD1a+ cells and S100+ dermal dendrocytes), and macrophages in skin lesions of a clinically well characterized NL group (N = 11). Results were compared to children (N = 23) and adults (N = 24) with classical tuberculoid leprosy.NL lesion histopathology was characterized by dense granulomatous inflammatory reaction, with a greater number of confluent tubercles when compared to the other groups. Neural compromise was seen in all biopsies. The frequency of Mycobacterium antigen was similar in all groups. The population of CD45RO+, CD4+ and CD8+ T lymphocytes, natural killer cells, B lymphocytes, CD1a+ epidermal cells, and macrophages of NL lesions did not differ from the other groups. The number of S100+ dermal dendritic cells of the NL group was smaller than that of the adult group, although it did not differ from the other group of children. Except for the confluent tubercules, our data could not disclose any other difference in the tissue reaction of NL, in spite of its peculiar clinical features and evolution when compared with the classical tuberculoid leprosy. The localization of NL lesions may be the result of the intimate skin contact with lepromatous parents or relatives, in areas such as cheeks, arms, buttocks, and limbs, and the innoculation of M. leprae into skin may strongly stimulate cell mediated immunity (CMI) against the bacilli. These circumstances might explain the good CMI response leading to high resistance, stability, and auto-resolution of nodular leprosy of childhood.
RÉSUMÉ
La lèpre nodulaire de l'enfance (LN) est une variante clinique bénigne de la lèpre tuberculoïde qui affecte les enfants en âge d'allaitement et des enfants qui sont restés dans un environnement à forte pression infectieuse. Les lésions cicatrisent complètement et la LN a été de ce fait considérée comme une manifestation d'allergie et d'immunité congénitale aux M. leprae. Nous avons étudié la réaction tissulaire in situ, la fréquence de détection des antigènes mycobactériens, les sous populations lymphocytaires CD45RO+, CD4+, CD8+, B et NK, ainsi que les cellules dendritiques (incluant les cellules épidermiques CD1a+ et les dendrocytes dermiques S100+) et les macrophages des lésions de la peau d'un groupe bien caractérisé de LN (N = 11). Les résultats ont été comparés à un groupe d'enfants (N = 23) et d'adultes (N = 24) souffrant de lèpre tuberculoïde classique.Les lésions histologiques de NL était composées d'une dense réaction inflammatoire granulomateuse et d'un plus grand nombre de tubercules confluents que ceux des autres groupes. Les lésions neurales ont été observées dans toutes les biopsies. La fréquence des antigènes anti-mycobactériens a été semblable dans tous les groupes. Les populations des lymphocytes T CD45RO+, CD4+, et CD8+, des cellules NK, des lymphocytes B, des cellules épidermiques CD1a+ et des macrophages des lésions de NL n'ont pas été différentes de celle des autres groupes. Les cellules dendritiques dermiques S100+ du groupe LN étaient moins nombreuses que celles du groupe d'adulte, bien qu'il n'ait pas de différence avec l'autre groupe d'enfants. A l'exception des tubercules confluents, nos données n'ont pu révéler aucune autre différence dans la réaction tissulaire de la NL malgré ses particularités et son évolution clinique, lorsque comparée avec celle de la lèpre tuberculoïde classique. Les localisations de la NL pourraient résulter de contacts intimes avec des parents lépromateux, en particulier les joues, les bras, les fesses et les jambes. Il est probable que l'inoculation de M. leprae dans la peau provoque une forte stimulation de l'immunité à médiation cellulaire (CMI) contre l'agent infectieux. Ces faits pourraient expliquer l'apparition d'une CMI vigoureuse, qui entraînerait une forte résistance, la stabilisation de la maladie et une résolution clinique spontanée de la lèpre nodulaire de l'enfance.
RESUMEN
La lepra nodular infantil (NL) es una variante clínica benigna de la lepra tuberculoide que afecta a los lactantes y a los niños que permanecen en regiones altamente infectadas. Las lesiones evolucionan con curación completa y la NL se ha llegado a considerar como una manifestación de alergia e inmunidad congénita contra Mycobacterium leprae. En este trabajo se estudió la reacción tisular, la frecuencia de antígenos micobacterianos, y la presencia de poblaciones de linfocitos (CD40RO+, CD4+, CD8+, B, NK), de células dendríticas (CD1a+ epidérmicas y dendrocitos dérmicos S100+), y de macrófagos en las lesiones de la piel de un grupo de pacientes NL bien caracterizado clínicamente (N = 11). Los resultados se compararon con los encontrados en niños (N = 23) y adultos (N = 24) con lepra tuberculoide (TT) clásica.Histopatológicamente las lesions NL estuvieron caracterizadas por la presencia de una densa reacción inflamatoria granulomatosa, con mayor número de granulomas confluentes que el observado en los otros grupos. En las biopsias de todos los casos se observó afección nerviosa. La frecuencia de antígenos micobacterianos fue similar en todos los grupos. Las poblaciones de linfocitos T CD45RO+, CD4+ y CD8+, células NK, linfocitos B, células epidérmicas CD1a+, y macrófagos en las lesiones NL no difirieron de los otros grupos. El número de células dendríticas S100+ en el grupo NL fue menor que en los adultos TT pero fue similar al encontrado en los niños TT. En comparación con las lesiones de la lepra TT, excepto por los granulomas confluentes, nuestros datos no revelan ninguna otra diferencia en la reacción tisular NL no obstante sus características clínicas peculiares y su evolución benigna. La localización de las lesiones NL puede ser el resultado del contacto íntimo de la piel con los progenitores o familiares lepromatosos, sobre todo en áreas expuestas como las mejillas, brazos, glúteos y piernas, donde la inoculación directa de M. leprae en la piel podría estimular fuertemente la inmunidad celular contra el bacilo, circunstancia que podría explicar la alta resistencia, la estabilidad, y la auto-resolución de la lepra nodular en los niños.
Nodular leprosy of childhood (NL) is a clinical variant of tuberculoid leprosy that affects infants and children exposed to a highly infected environment, such as those born to lepromatous parents living in leprosy sanatoriums in the past. Lara and De Vera (1935) first described this clinical variant of leprosy among very young children born at Culion Sanatorium in the Philippines. The authors pointed out a marked tendency of the disease to subside, with or without treatment (16). In Brazil, Souza Campos was the first to present a comprehensive clinical, immunological and histologic characterization of NL among breast-fed children from leprosy patients. He emphasised the benign nature of this form of primary tuberculoid leprosy in children (30). Indurated papulonodules, nodules, wheel-like lesions, raised macules, solitary infiltration, and lichenoid skin lesions characterize NL. The lesions occur commonly in small numbers and are frequently solitary and localized in skin areas usually submitted to close contact with infected areas of sick relatives (cheeks, arms, limbs, and buttocks) (13,22,30). NL lesions resolve with spontaneous healing, leaving a quite characteristic scar (13,31). There are no peripheral neural involvement or deformities (31). The lepromin skin test is strongly positive (22,30,31), and there is some controversy about the demonstration of acid-fast bacilli (AFB) in the lesions (17).
Souza Campos (1937) considered NL lesions to be manifestations of allergy and immunity (30). Nolasco and Lara reported complete healing in most cases, indicating a high degree of resistance. The children observed had remained in a highly infected environment throughout their illness without presenting new signs of disease (22).
In spite of its peculiar clinical evolution, the histopathologic features of skin lesions are described as similar to that observed in classical tuberculoid leprosy, showing well formed epithelioid cell granulomata in the dermis (22,23,26,30).
Appropriate cellular mediated immunity is essential for host protection against Mycobacterium leprae infection (3,9,10,18,28). Immunohistochemical techniques allow the evaluation of phenotypes of cellular elements in tissue inflammatory infiltrates (10,19,33), enhancing immunopathologic observations of the host-parasite relationship.
Since there are no data on immunopathologic aspects of the tissue reaction that could be related to the peculiar clinical evolution of NL, we described and quantified the cellular elements related to cell mediated immunity, as well as Mycobacterium antigens, in skin biopsies of NL, comparing these results to those observed in classical tuberculoid leprosy of children and adults.
MATERIALS AND METHODS
Skin biopsies were obtained from 11 patients with clinical diagnosis of NL, and from 23 children and 24 adult patients with classical tuberculoid leprosy.
Eleven infants and children (3 males and 8 females) constituted the NL group (group 1), with ages ranging from 19 months to 9 yrs (mean of 3.61 yrs). The tuberculoid leprosy children group (group 2) was composed of 23 patients (9 males and 14 females), with ages ranging from 2 to 14 years of age (mean of 8.63 yrs). The adult group (group 3) included 24 patients (11 male and 13 female) with ages ranging from 19 to 69 yrs (mean of 40.33 yrs). The Mitsuda skin test was positive in all patients.
Nine NL patients were born to lepromatous mothers, and the remaining had close relatives (one father and one aunt) with lepromatous disease. We could access their complete clinical records and follow up (mean duration of 5.9 yrs), and all patients presented an auto-resolutive course of disease without specific treatment. Only one patient received chaulmoogra oil treatment. All of them resolved without progression to other disease forms or deformities.
Five µm tissue sections obtained from each skin specimen were submitted to routine histological procedures and stained with hematoxylin-eosine and Fite-Faraco technique for AFB. The histopathologic features analyzed included the inflammatory infiltrate pattern, nerve involvement, and epidermal changes.
In order to demonstrate T lymphocytes (Pan T cells, CD4 and CD8 lymphocytes), natural killer cells, B lymphocytes, macrophages, Langerhans cells, nerves, and mycobacteria antigens, 5 µm histologic sections were placed on glass slides coated with 3-aminopropyltrietoxy-silane (Sigma A3648) and submitted to immunohistochemical staining techniques with streptABC complex/HRP (Dako 0492), according to Hsu, et al. (1981) (8). Monoclonal antibodies used were anti-CD45RO (Dako M742) at a dilution of 1:100, anti-CD4 (Dako M834) at a dilution of 1:100, anti-CD8 (Dako M7103) at a dilution of 1:50, anti-CD56 (Becton-Dickison HNK1) at a dilution of 1:800, anti-CD20 (Dako M755) at a dilution of 1:100, anti-CD68 (DAKO M876) at a dilution of 1:50, anti-S100 protein (Biogenex 15E2E2) at a dilution of 1:1000, anti-CD1a (Immunotech 1590) at a dilution of 1:20, and polyclonal antibody rabbit anti-Mycobacterium bovis (Dako B124) at a dilution of 1:60,000. Reactions using anti-CD4, anti-CD8, and anti-CD1a antibodies were performed with the Catalyzed Signal Amplification (CSA) method (Dako K1500) (11). Reactions using anti-CD68 and anti-CD56 antibodies were performed after antigen heat retrieval in citrate buffer 0.01 M pH 6.0 in a micro-oven and pressure cooker, respectively. The reactions were developed with chromogen 3-3-diaminobenzidine (Sigma) and counterstained with Mayer's hematoxylin.
Lymph node fragments and lepromatous leprosy skin biopsies were used as positive controls for the immunohistochemical techniques with the anti-cellular and anti-BCG antibodies, respectively. Negative controls were obtained by omitting the primary antibodies for each reaction; they were replaced by saline phosphate buffer solution (0.01 M pH 7.4).
The number of T lymphocytes (CD45RO+ cells), CD4+ and CD8+ T lymphocytes, natural killer cells (CD56+ cells), S100-protein positive dendritic dermal cells, and CD68 positive cells were obtained by counting the number of stained cells in nine 0.0625 mm2 randomized microscopic fields.
Quantification of epidermal Langerhans cells (CD1a positive cells) was obtained by evaluation of CD1a+ epidermal expression. It was obtained by using a x 10 ocular with a grid with 100 hits (i.e., points of crosses of two lines) and a x 40 objective. The fraction of area of epidermis positive for CD1a antigen was obtained by counting the number of hits over CD1a positive reaction and the total number of hits over the entire epidermis (excluding the cornified epidermal layer) of each specimen. The ratio of the number of hits over CD1a positive reaction/total number of hits over the epidermis corresponded to the epidermal fraction of area occupied by Langerhans cells (2).
Bacillary antigens, as well as AFB, were verified in the whole dermis of each skin fragment stained with anti-Mycobacterium bovis antibody and stained with Fite-Faraco technique.
Numerical data obtained for each group were compared by non-parametric statistical tests of Kruskal-Wallis and Mann-Whitney. The frequency of observation of confluent granulomas, Mycobacterial antigen, and AFB among the three groups was compared by the Fisher's exact test. The level of significance was set at p 0.05.
RESULTS
All three groups studied exhibited the same histopathologic features. It was characterized by compact epithelioid tubercles surrounded by lymphocytes and few plasma cells. Langhans giant cells were seen in almost all specimens. Inflammatory reaction obscured the dermal-epidermal interface. Epithelioid granulomas were elongated and involved superficial and deep dermal vessels, cutaneous adnexal structures, small dermal nerves, and arrector pili muscles. Confluent granulomas were seen in 8/11 biopsies from group 1 (Fig. 1 ), 5/23 from group 2, and 5/24 from group 3. The number of skin specimens showing confluent granulomas in group 1 was significantly higher than those seen in groups 2 and 3 (p <0.05).
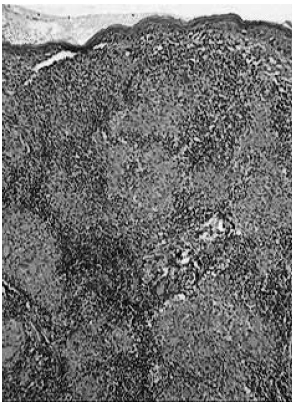
Fig. 1. Nodular leprosy of childhood. Confluent granulomas with compact epitheliod cell clusters surrounded by lymphocytes and Langhans giant cells.
(Hematoxylin and eosin – original magnification ×40).
Nerve lesions were better disclosed by S100 protein reaction, which revealed nerve fragments within granulomas (Fig. 2 ) in all groups studied.
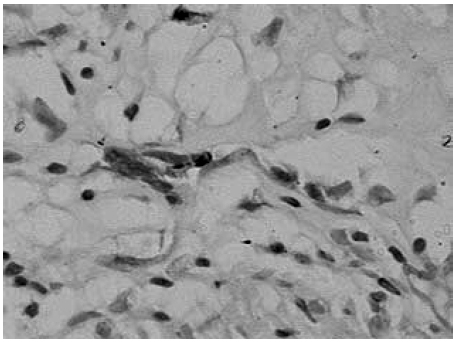
Fig. 2. Nodular leprosy of childhood. Fragmented nerve inside a granuloma detected by S-100 protein. (Streptavidin-biotin peroxidase complex – original magnification ×400).
T lymphocytes (CD45RO+ cells) were the predominant small mononuclear cells of the dermal infiltrate, and were observed within the whole granuloma formation. CD4+ T lymphocytes exhibited the same distribution as CD45RO+ T lymphocytes, while CD8+ T lymphocytes showed a tendency to surround the epithelioid tubercles. Few T lymphocytes of either phenotype were observed within the basal and parabasal layers of epidermis. Natural killer cells revealed by anti-CD56 antibody were large round cells distributed sparsely within granulomata. B lymphocytes were scarce and arranged in small clusters around epithelioid tubercles. Compact clusters of epithelioid cells as well as macrophages placed among the other inflammatory cells were strongly stained by anti-CD68 antibody. S100 protein positive dermal dendritic cells (dermal Langerhans cells) were seen at the periphery and around granulomas.
Epidermal Langerhans cells were better shown by anti-CD1a antibody. They were regularly distributed among keratinocytes and exhibited long and anastomosing dendrites in the three groups (Fig. 3).
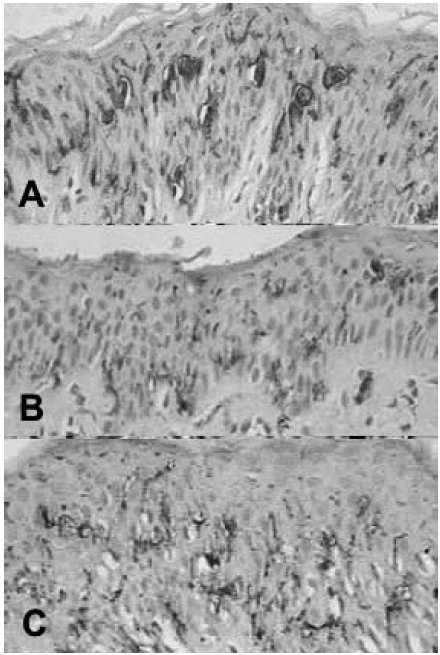
Fig. 3. Nodular leprosy of childhood (A) and tuberculoid leprosy in children (B) and adults (C). CD1a+ Langerhans cells within the epidermis. (Streptavidin-biotin peroxidase complex – original magnification ×400).
AFB were seen in 18.18% of group 1 specimens, 8.69% of group 2, and 16.66% of group 3. The anti-Mycobacterium bovis immune reaction revealed Mycobacterial antigen in 72.72% of group 1 specimens, 60.86% of group 2, and 58.33% of group 3. There was no difference in the frequency of AFB nor mycobacterial antigens between the three groups studied.
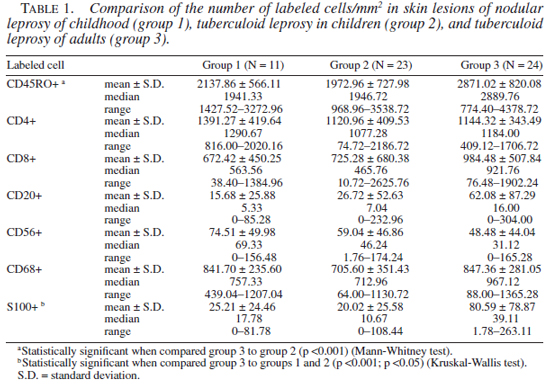
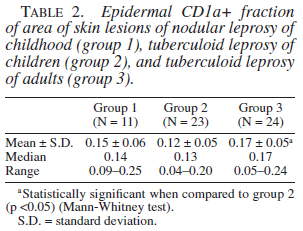
The number of T lymphocytes (CD45RO positive cells) and its subsets (CD4+ and CD8+ T lymphocytes), natural killer cells, B lymphocytes, macrophages, and epidermal Langerhans cells population of group 1 did not differ from the other two groups. Group 3 showed a higher number of T lymphocytes and epidermal Langerhans cells when compared to group 2 (p <0.001 and p <0.05, respectively). The number of dermal dendritic cells revealed by anti-S100 protein antibody in group 1 and group 2 biopsies were smaller than that in group 3 (p <0.05 and p <0.001, respectively). Results of quantification of these different immunolabeled cells are presented in Tables 1 and 2.
DISCUSSION
NL was first described by Souza Campos (30). It is considered a benign form of tuberculoid leprosy that affects breast-feeding children and infants that remained in a highly infected environment. Lesions resolve usually to complete healing, and NL is considered to be a manifestation of allergy and congenital immunity to M. leprae (31). Other authors that referred to spontaneous healing in childhood leprosy (13,14,17) confirm the essentially benign character of papulonodular leprosy.
The epidemiological and clinical presentation of patients in group 1 were similar to that related by former authors (15,16,17,22,30). Mean age of patients was 3.61 years. All cases presented positive Mitsuda reaction, 9 of them were born to lepromatous mothers, and 2 were living with close relatives who had lepromatous disease. All patients with nodular lesions resolved to healing without disease progression or deformities.
Histopathology of NL lesions was characterized by dense granulomatous inflammatory reaction with confluent tubercles. Confluent granulomas were more frequently seen in group 1 skin specimens than in groups 2 and 3. Probably the nodular aspect of these lesions was a macroscopic translation of this microscopic feature. Neural injury was present in all biopsies from group 1.
According to Nolasco and Lara (22), nodular leprosy lesions exhibit predominantly tuberculoid histology. These authors stated that tuberculoid foci were usually not as well defined as those seen in tuberculoid lesions of older children and adults. They also reported that, in skin lesions, some of the nerve twigs may exhibit tuberculoid type of infiltration, as well as thickened cellular and laminated epineurium.
In all three groups studied neural damage was better demonstrated by S100 protein immune staining, which disclosed nerves fragments inside granulomas. S100 protein staining was considered to be superior to hematoxylin and eosin staining in identifying dermal nerve destruction in leprosy (6,21,32). In spite of neural damage seen in skin lesions, patients with nodular leprosy resolve without peripheral nervous system injury such as neuritis, amyotrophy, paralisys, or trophic lesions (1,4).
AFB and Mycobacterium antigen frequency in lesions were similar in the three groups studied. Immunohistochemistry sensitivity to detect bacterial antigen was greater than that of Fite-Feraco staining. We observed AFB in 18.18% of NL biopsies and Mycobacterium antigen in 72.72% of the specimens. Nolasco and Lara (13) demonstrated AFB in nearly 75% of their cases, although Souza Campos (30) remarked on the scarcity or absence of AFB in the lesions of nodular leprosy.
The development of an effective immune response to invading pathogen is achieved by the complex interaction of a variety of immuno-competent cells including T cells, B cells, natural killer cells, and macrophages (7). T lymphocytes play a key role in managing the immune response against M. leprae as it avoids dissemination of bacteria and, consequently, of disease (3,24). T-cell-dependent immunity to M. leprae is high in healthy exposed individuals and in tuberculoid leprosy patients (24). In NL, as in the other two groups studied, we observed T lymphocytes and CD4+ lymphocytes within epithelioid granulomas. CD8+ T lymphocytes were seen surrounding epithelioid tubercles. The distribution of T lymphocytes and their subsets was the same as described by Modlin, et al. (19) in tuberculoid leprosy lesions. The authors correlated the intimate admixture of CD4+ T lymphocytes and epithelioid histiocytes to a possible co-operation in promoting effective immune response. They also speculated that CD8+ T lymphocytes at the periphery of granulomas might contribute to bacilli confinement within circumscribed granulomas.
In our study, CD45RO+, CD4+ and CD8+ T lymphocyte, natural killer cell, B lymphocyte, and macrophage populations in NL lesions did not differ from tuberculoid leprosy groups. The role of B lymphocytes in leprosy is not totally clear, but in tuberculoid lesions its population is higher than in lepromatous leprosy lesions (5).
Dendritic cells, monocytes, and natural killer cells play a role in shaping the nature of the T cell response to a pathogen (7). We could not demonstrate any difference in the natural killer cell population, as well as of macrophages in NL skin lesions when compared to the tuberculoid leprosy groups studied.
The population of epidermal Langerhans cells demonstrated by CD1a immune stain in NL group did not differ from that seen in the other groups. The Langerhans cell population and distribution in the epidermis of tuberculoid leprosy lesions is described as comparable to that of normal skin (29). S100 protein staining was also used to demonstrate dermal dendritic cells. These cells were large with prominent dendrites, and observed at the periphery of granulomas. This feature was described in tuberculoid leprosy when dendritic cells are labelled with anti CD1a, -b, and -c monoclonal antibodies on fresh frozen tissue specimens (29). Anti-CD1a antibody used in paraffin-embedded tissue only stained epidermal Langerhans cells. The number of S100 protein positive dermal dendritic cells of NL group was smaller than that of the adult tuberculoid leprosy group (group 3), although it did not differ from the other group of children with tuberculoid disease. Dendritic cells are regarded as major stimulators of primary T cell responses, as well as potent stimulators of memory responses. There is evidence of a link between the response of the innate immune system to the acquired T cell-mediated response through the induction of a population of potent antigen presenting cells in leprosy infection (29).
Except for the confluent tubercules, our data could not disclose any other difference in the tissue reaction of NL, in spite of its peculiar clinical features and evolution when compared with the classical tuberculoid leprosy.
It has been suggested that the clinical spectrum of leprosy is incomplete in the 0-14 years age group, and a discrepancy between clinical and histologic parameters has been reported in these patients (12,27). Nodular leprosy may be an exception of children response against M. leprae correlated with its clinical evolution and tissue reaction, represented by well organized epithelioid granulomas.
There are many factors influencing the development of leprosy. The clinical spectrum is related to the ability of the patient's cell mediated immune response (CMI) in destroying M. leprae. An innate immunity has been proposed for some of the infected individuals; however, for the majority of the patients CMI seems to be the predominant factor. The transmission of infection is still a point of discussion. It has been suggested that the entry point of M. leprae may be important to the immune response. An encounter at skin and draining lymph nodes (peripheral compartment) stimulates CMI (20). Localization of NL lesions are described as those areas of closest contact with lepromatous parents or relatives, such as cheeks, arms, buttocks, and limbs (13,22,30), suggesting that the inoculation of M. leprae into the skin in this group of patients may strongly stimulate CMI against the bacilli. These circumstances might explain a good CMI response leading to high resistance, stability, and auto-resolution of nodular leprosy of childhood.
Acknowledgments. We thank Dr. José Pessoa Mendes (in memoriam) who allowed us to consult his personal records and follow up of NL patients, and Carla Pagliari for the immunohistochemical techniques.
REFERENCES
1. Bechelli, L.M. and Pagano, P.M.G. Reflections on some aspects of leprosy among children in Brazil and in other countries. Acta Leprol. 7(1990)229-237.
2. Bieber, T., Ring, J. and Braun-Falco, O. Comparison of different methods forenumeration of Langerhans cells in vertical cryosections of human skin. Br. J. Dermatol. 118(1998)385-392.
3. Britton, W.J. Leprosy 1962-1992 Immunology of leprosy. Trans. Roy. Soc. Trop Med. Hyg. 87(1993)508-514.
4. Cabrera, H.N., Luppi, M.S. and Ferrer, F. Lepra nodular de la infancia. Leprologia. 18(1973)268-270.
5. Finiasz, M.R., Arias, D.A., Valdez, R., Estevez, M.E. and Sem, L. Subpoblaciones linfoides T y B en enfermos con lepra. Medicina(Buenos Aires). 41(1981)131-136.
6. Fleury, R.N. and Bacchi, C.E. S-100 protein and immunoperoxidase technique as a first aid in the histopathologic diagnosis of leprosy. Int. J. Lepr. Other Mycobact. Dis. 55(1987)338-344.
7. García, V.E., Uyemura, K., Sieling, P.A., Ochoa, M.T., Morita, C.T., Okamura, H., Kurimoto, M., Rea, T.H. and Modlin, R.L. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J. Immunol. 162(1999)6114-6121.
8. Hsu, S.M., Raine, L. and Fanger, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedure. J. Histochem. Cytochem. 29(1981)577-580.
9. Jullien, D., Sieling, P.A., Uyemura, K., Mar, N.D., Rea, T.H. and Modlin, R.L. IL-15, an immunomodular of T cell responses intracellular infection. J. Immunol. 158(1997)800-806.
10. Kaplan, G. and Cohn, Z.A. The Immunobiology of leprosy. Int. Rev. Exp. Path. 28(1986)45-78.
11. Key, M.E. and Phillips, T. Catalized signal amplification (CSA). An enhanced immunohistochemical staining method based on biotinyl tyramide. J. NIH Res. 8(1996)72
12. Kumar, B., Rani, R. and Kaur, I. Childhood leprosy in Chandigarh: clinico-histopathological correlation. Int. J. Lepr. Other Mycobact. Dis. 68(2000)330-331.
13. Lara, C.B. Early leprosy in children of lepers. Further observations on the arly definitely leprotic lesions. Monthly Bull. Bureau Health. 23(1938)325-350.
14. Lara, C.B. Leprosy in children. General considerations: initial and early stages. Acta Leprol. 38(1970)29-60.
15. Lara, C.B. and de Vera, B. Clinical observations with reference to leprosy in children of lepers. J. Phil. Isl. Med. Ass. 15(1935)115-129.
16. Lara, C.B. and de Vera, B. Early leprosy in infants born of leprous parents, with report of cases. J. Philip. Isl. Med. Ass. 15(1935)252-260.
17. Lara, C.B. and Nolasco, J.O. Self-healing, or abortive, and residual forms of childhood leprosy and their probable significance. Int. J. Lepr. 24(1956)245-263.
18. Mehra, V. and Modlin, R.L. T-lymphocytes in leprosy lesions. Curr. Top. Microbiol. Immunol. 155(1989)97-109.
19. Modlin, R.L., Hoffman, F.M., Tayor, C.R. and Rea, T.H. lymphocyte subsets in the skin lesions of patients with leprosy. J. Am. Acad. Dermatol. 8(1983)182-198.
20. Naafs, B., Silva, E., Vilani-Moreno, F., Marcos, E.C., Nogueira, M.E. and Opromolla, D.V.A. Factors influencing the development of leprosy, an overview. Int. J. Lepr. Other Mycobact. Dis. 69(2001)26-33.
21. Narayan, R., Maheswari, P.K. and Desikan, K.V. Detection of S-100 protein and anticeramide antibodies in leprosy patients by ELISA. Lepr. Rev. 68(1997)117-124.
22. Nolasco, J.O. and Lara, C.B. Histopathology of early lesions in fourteen children of lepers. Analysis of previous skin blemishes in relation to sites of biopsies and other positives and probable lesions. Philip. J. Sci. 71(1940)321-358.
23. Noussitou, F.M. Lepra infantil. Organizacion Mundial de la Salud, Ginebra. (1976)
24. Ottenhoff, T.H. Immunology of leprosy: lessons from and for leprosy. Int. J. Lepr. Other Mycobact. Dis. 62(1994)108-121.
25. Pagliari, C., Duarte, M.I.S. and Sotto, M.N. Pattern of Mycobacterial antigen detection in leprosy. Rev. Inst. Med. Trop. São Paulo. 37(1995)7-12.
26. Schujman, S. and Fernandez, J.M.M. Cicatriz residual da lepra tuberculóide infantil. Rev. Bras. Lepr. 9(1941)337-350.
27. Sehgal, V.N., and Joginder. Leprosy in children: correlation of clinical, histopathological, bacteriological, and immunological parameters. Lepr. Rev. 60(1989)202-205.
28. Sengupta, U. Cell-mediated immunity in leprosy: an update. Int. J. Lepr. Other Mycobact. Dis. 61(1993)439-454.
29. Sieling, P.A., Jullien, D., Dahlem, M., Tedder, T.F., Rea, T.H., Modlin, R.L. and Porcelli, S.A. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J. Immunol. 162(1999)1851-1858.
30. Souza Campos, N. Aspects cliniques de la lèpre tuberculoïde chez l'enfant. Rev. Bras. Lepr. 5(1937)33-113.
31. Souza Lima, L. and Souza Campos, N. Lepra tuberculóide. Editora Renascença. São Paulo. (1950)
32. Thomas, M.M., Jacob, M., Chandi, S.M., George, S., Pulimood, S., Jeyaseelan, L. and Job, C.K. Role of S100 staining in differentiating leprosy from other granulomatous diseases of skin. Int. J. Lepr. Other Mycobact. Dis. 67(1999)1-5.
33. Van Voorhis, W.C., Kaplan, G., Sarno, E.N., Horwits, M.A., Steinmen, R.M., Lewis, W.R., Nogueira, N., Hair, L.S., Gattas, C.R., Arrick, B.A. and Cohn, Z.A. The cutaneous infiltrates of leprosy: cellular chracteristics and the predominant T cell phenotypes. N. Engl. J. Med. 307(1982)1593-1597.
1. M.D., Ph.D., Department of Pathology;
2. M.D., Ph.D., Departments of Pathology and Dermatology, University of São Paulo Medical School;
3. M.D., São Paulo Health Secretary;
4. M.D., Ph.D., Department of Dermatology, University of São Paulo Medical School, São Paulo, Brazil.
Reprint Requests to: Mírian N. Sotto, M.D., Avenida Dr. Arnaldo 455 sala 1118, CEP 01246-903 São Paulo-SP Brazil. FAX: (55-11) 3063-0718, E-mail: mnsotto@ajato.com.br
Received for publication on 5 March 2003.
Accepted for publication on 13 May 2003.