- Volume 71 , Number 3
- Page: 231–9
Electron microscopic appearance of lepromatous foodpads of nude mice1
ABSTRACT
Footpad lesions of 3 nude mice infected by Mycobacterium leprae were studied at 9, 12, and 14 months after inoculation with light and electron microscope. The lesions were somewhat similar to those found in nodules in polar lepromatous leprosy. Striated muscles rather than nerves were the preferred site of the growth of M. leprae. Yet, M. leprae were identified in Schwann cells and endothelial cells, singly and in clumps. M. leprae filled macrophages, and free M. leprae were found in large numbers in the endoneurium without producing any significant demyelination.RÉSUMÉ
Les lésions de la plante des pieds de 3 souris nues infectées par Mycobacterium leprae furent étudiées à 9, 12 et 14 mois après inoculation en microscopie optique et électronique. Les lésions étaient très similaires à ce qui a été rapporté dans les nodules de lèpre lépromateuse. Les muscles striés plutôt que les nerfs étaient les sites principaux de la multiplication des M. leprae. Cependant les M. leprae furent identifiées dans les cellules de Schwann et dans les cellules endothéliales, isolées ou en amas. Les macrophages étaient emplis de M. leprae, et des M. leprae extracellulaires ont été trouvés en grand nombre dans l'endoneurium, sans la production d'une démyélinisation significative.RESUMEN
Se realizó un estudio por microscopía de luz y electrónica de las lesiones inducidas por Mycobacterium leprae en la almohadilla plantar de 3 ratones desnudos a los 9, 12 y 14 meses de la inoculación. Las lesiones fueron en cierta forma similares a las encontradas en los nódulos de la lepra lepromatosa polar. Aunque los músculos estriados más que los nervios, fueron el sitio de crecimiento preferido de M. leprae también se observaron bacilos, aislados y agrupados, en las células de Schwann y en las células endoteliales. Así mismo se encontraron grandes números de macrófagos repletos de M. leprae y bacilos aislados en el endoneurio, sin evidencias de desmielinización significativa.It was in 1960 that Shepard first demonstrated the growth of Mycobacterium leprae localized in the footpads of normal mice (1). He based his experiments-that M. leprae preferred to multiply in areas cooler than the normal body temperature-partly on the observations of Binford (1) and Brand (2). Later, in 1976, markedly enhanced growth of the organisms was reported in footpads of nude mice (4); in addition, the infected nude mice developed systemic disease and the disease spread to the internal organs such as liver, spleen, lungs, and lymph nodes. Since then, the mouse footpad models have been used for the study of pathology and pathogenesis of leprosy, for testing the efficacy of anti-leprosy drugs, for screening M. leprae for drug resistance, and for the production of M. leprae for use in experimental studies. The histopathological and electron microscopic appearances of the nude mice footpad lesions were first published in 1983, and the occurrence of generalized lesions in the animal was emphasized (3). In this presentation, an attempt is made to compare the lesions in the footpads of nude mice to those in lepromatous patients.
MATERIALS AND METHODS
Biopsies were obtained from footpads of 3 nude mice infected with 107 M. leprae, 6, 12, and 14 months after infection. The biopsies were immediately divided into 2 parts, and one piece was fixed in 10% buffered formalin and processed for light microscopic studies, while the other piece was minced into 1 mm cubes, fixed in 5% glutaraldehyde, and processed for electron microscopic studies.
Formalin-fixed tissues were dehydrated and embedded in paraffin. Five micron sections were cut and stained with routine hemotoxylin and eosin stain and a modified Fite's stain for M. leprae (7). The sections were examined under a light microscope. The 1 mm cubes of the infected footpad were fixed in 5% glutaraldehyde and, after processing, were embedded in araldite; 1 micron thick sections were first made of all blocks and stained with toludine blue for the orientation of the tissue. Blocks that contained nerves and muscle bundles were specifically looked for and selected. Ultra thin sections were made and stained with uranyl acetate and lead citrate, and were examined using a Philips EM 410 transmission electron microscope.
RESULTS
Light microscopic studies. On examination, large collections of macrophages with abundant cytoplasm dominated the histopathologic picture. A clear zone, uninvolved by the granuloma, was present immediately below the epidermis. The granuloma surrounded, infiltrated, and destroyed striated muscles, tendons, and hair follicles. Nerves and blood vessels and the small bones of the foot were also surrounded by the granuloma. Scattered lymphocytes, a few focal collections of plasma cells, and occasional mast cells were present. Several small focal clumps of polymorphs were seen throughout the infiltration. Although the striated muscle bundles were extensively destroyed by the infiltration of macrophages, the structure of the nerve bundles was not compromised as the granuloma of macrophages only surrounded the bundles, and the perineurium appeared to remain intact. The population of inflammatory cells of the lepromatous footpad found at 12 and 14 months was essentially similar. However, the size of the granuloma and the number of macrophages gradually increased with the aging of the lesions. Further, there was a gradual change in the cytoplasm of the macrophages. While most of these had a pink granular cytoplasm at 6 months, there was an increasing foamy change and vacuolation of the cytoplasm as they aged. The infiltration of the muscles and tendons by the granuloma was significant at 14 months, and only remnants of them were identified. Acid-fast stains showed large clumps of acid-fast bacilli (AFB) inside macrophages and muscle cells. Smaller numbers of AFB were found in nerves and endothelial cells. As the lesions aged, AFB became granular, and at 14 months a large majority of them were fragmented. The presence of AFB inside nerve bundles was easily evident at 12 and 14 months with the invasion of M. leprae into the peri- and endoneurium.
Electronmicroscopic studies. Macrophages with groups of M. leprae were seen in large numbers (Fig. 1). Although a large majority of the organisms appeared to be intact in cross sections, the specimens examined at 14 months showed an increase in fragmentation of the cytoplasm and collapse of the cell membrane of M. leprae. Electron transplant zones (ETZ) were present around single organisms and around collections of bacilli. The amount of ETZ increased with the aging of the lesions. Very occasionally mast cells, plasma cells, and lymphocytes were seen. The sections of nerve bundles showed perineurium with well-preserved perineurial cells. Collagen fibrils were found investing the perineurial cells, and occasional bacilli were found within the cells (Fig. 2 ). There were endoneurial macrophages packed with M. leprae in close proximity to Schwann cells containing myelinated axons (Fig. 3 ), but no organisms were present in the Schwann cells. Over 100 myelinated axons in the cross sections of two nerve bundles were scrutinized and, although structural changes of the myelin attributable to fixation artifacts were present, there were no obvious demyelinating changes, except one. The axon in that Schwann cell showed marked demyelination and signs of degeneration. A structure resembling M. leprae was incorporated into the degenerated myelin (Fig. 4 ). The cytoplasm of several Schwann cells containing myelinated axons was thrown into many papillary folds, forming numerous processes. These Schwann cells contained a few bacilli (Fig. 5 ) or clumps of M. leprae (Fig. 6 ) with EZT around them. Blood vessels and capillaries were intact, but there was some swelling of endothelial cells. The endothelial cells of a few lymphatic vessels contained several M. leprae (Fig. 7 ). Striated muscle cells were infiltrated by bacilli with evidence of homogenization and destruction of the myofibrils (Fig. 8 ). A very few fibroblasts were present, and there were collections of collagen fibrils in the endo- and perineurium. Many mitochondria, dense bodies, and M. leprae were present in the interstitial tissue, possibly released from degenerating macrophages.
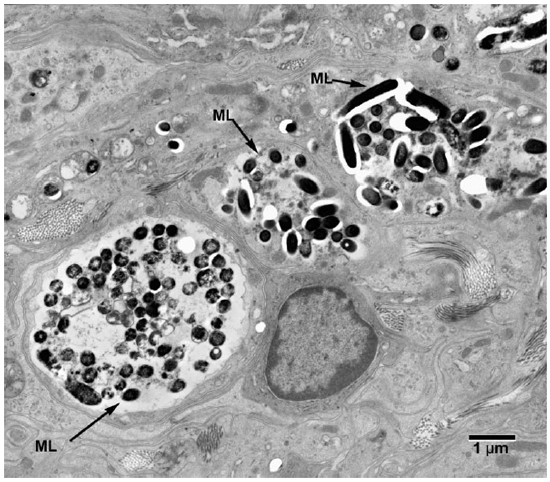
Fig. 1. Electronmicrograph (EM) to show colonies of M. leprae in macrophages. (ML = M. leprae)
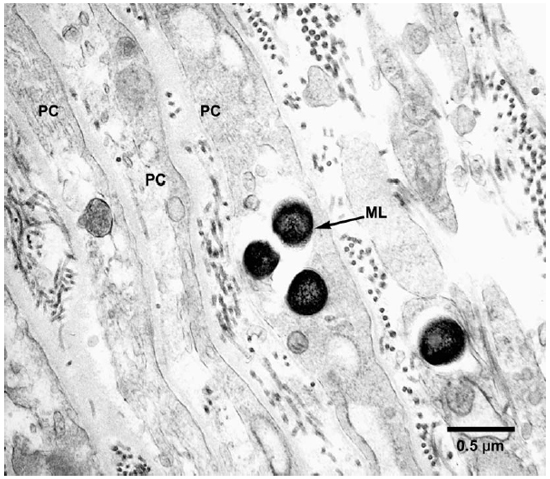
Fig. 2. EM to show perineurial cells invested by collagen fibrils and containing M. leprae. (PC = Perineurial cells, ML = M. leprae)
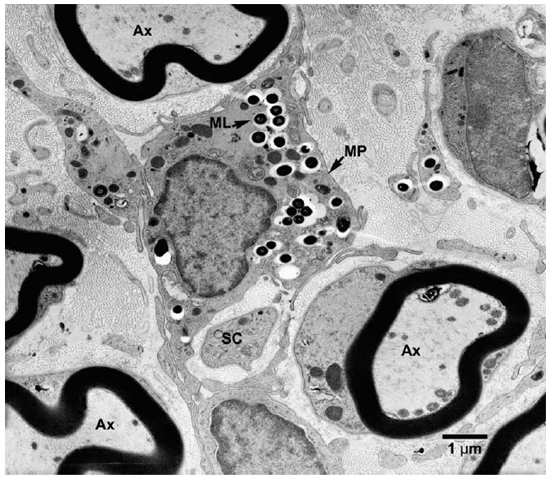
Fig. 3. EM of an endoneurial macrophage containing numerous M. leprae. (MP = macrophage, Ax = Axon, SC = Schwann cell)
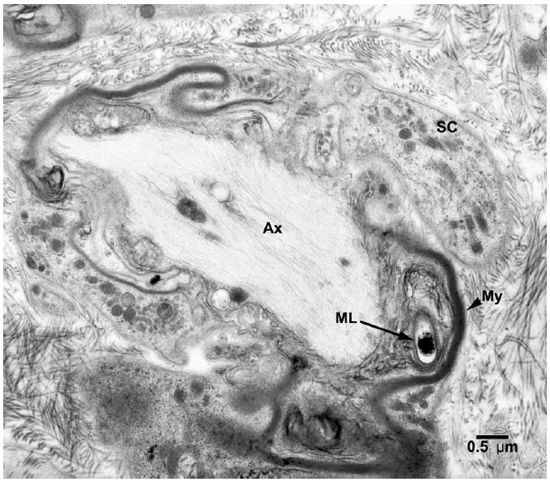
Fig. 4. EM of a Schwann cell containing a demyelinated and degenerating axon with one M. leprae incorporated in the myelin. (SC = Schwann cell, Ax = Axon, ML = M. leprae)
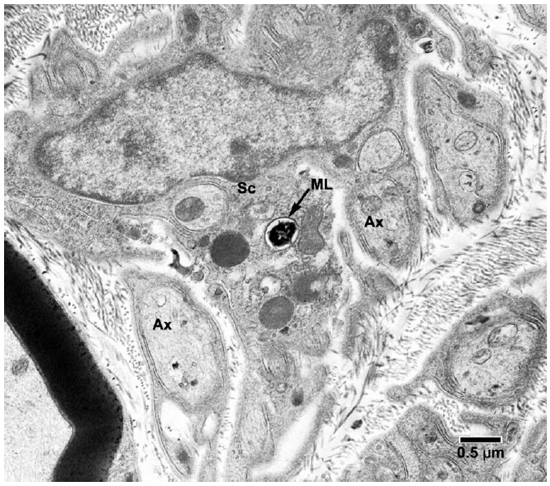
Fig. 5. EM of a Schwann cell of a non-myelinated axon containing one M. leprae. (ML = M. leprae, SC = Schwann cell, Ax = Axon)
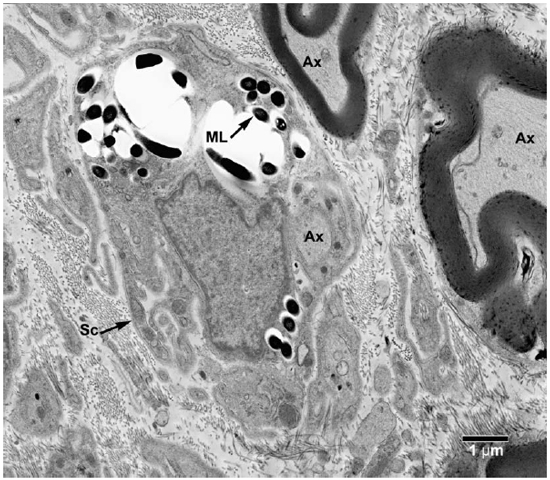
Fig. 6. EM of a Schwann cell with its processes containing clumps of M. leprae. (Ax = Axon, ML = M. leprae, SC = Schwann cell)
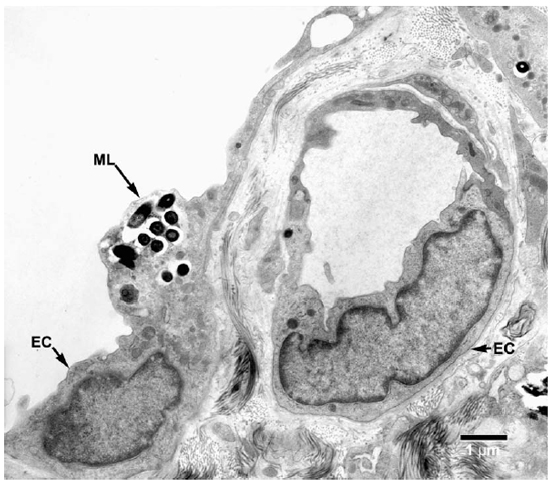
Fig. 7. EM of an endothelial cell of a lymphatic vessel containing a small collection of M. leprae. (ML = M. leprae, EC = Endothelial cell).
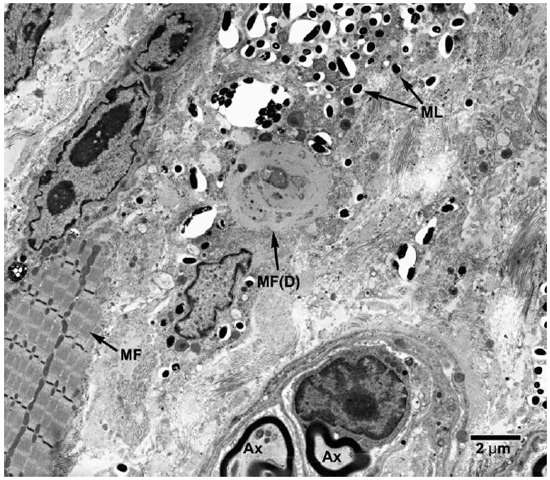
Fig. 8. EM of striated muscle cells undergoing degenerative changes following invasion by M. leprae. (ML = M. leprae, MF = Myofibrils, MF(D) = degenerating muscle tissue).
DISCUSSION
The lepromatous nodule in the footpad of the nude mouse is very similar in its histologic and electron microscopic appearances to those of a skin nodule in polar lepromatous leprosy (LL) of humans (6). The granuloma seen in the footpads of nude mice consists entirely of macrophages with a scant sprinkling of lymphocytes, plasma cells, and mast cells, surrounding the muscles, tendons, nerves, and blood vessels. The gradual accumulation of intracellular lipids around M. leprae, giving rise to a vacuolated appearance of the macrophages, increases with the increasing age of the granuloma and is associated with fragmentation and granularity of intracellular AFB. The progressive degenerative changes seen among M. leprae in the late stage infected nude mouse footpad are consistent with the decreased viability of these organisms that has been observed in radio respirometry assays (14). As the lesions advance, the muscles, tendons, and nerves are infiltrated and partly destroyed. In lepromatous leprosy (LL) in humans, initially the granuloma surrounds the adnexal structures, and later they are infiltrated and destroyed.
However, there are differences in the involvement of peripheral nerves. Although histopathological appearance shows endoneurial clumps of bacilli in nude mice, in the ultrastructural study most of the large colonies of M. leprae are not in Schwann cells, but found in endoneurial macrophages. A small number of Schwann cells of nonmyelinated fibers contained M. leprae in varying numbers. Schwann cells of nonmyelinated axons in humans harbored as many M. leprae as found in macrophages, and those of myelinated fibers were also invaded by M. leprae but to a lesser extent. Perineurial cells of nude mice were invaded by M. leprae, similar to the appearance in humans. As pointed out in an earlier study, we confirm that in experimental animals, the peripheral nerve is not the preferred site of M. leprae (8), although features of neural involvement in armadillos are similar to those in man (10,11). In mice, it is highly likely that the striated muscle is the preferred site for growth of M. leprae.
In a recent study of the sciatic nerve in mice, it is stated that intraneural inoculation failed to produce Schwann cell invasion by M. leprae. The injected organisms at the site gradually degenerated and the lesions regressed (13). It is well known that the growth of M. leprae is temperature-dependent, and tissues with higher temperatures do not sustain and support their growth. In a study on transmission of leprosy using T900 (thymectomized, irradiated) mice, M. leprae failed to grow when injected at their flanks, but produced local multiplication and dissemination to other parts of the body when inoculated into the footpad (Ebenezer, G. 2003. Personal communication.) It was suggested that this difference is because of the higher temperature at the flanks.
In a recent publication it was stated that in an in vitro nerve tissue culture, M. leprae induced demyelination upon contact with Schwann cells containing myelinated fibers (9). In the present study it was found that Schwann cells containing many myelinated axons were in close contact with bacilli-packed intraneural macrophages and with M. leprae released from macrophages, found free in the interstitial tissue. Over 100 myelinated axons were closely scrutinized with the electron microscope. Except in one, there was no evidence of active demyelination in any of them. In an earlier ultrastructural study of radial cutaneous nerves obtained from lepromatous patients, several Schwann cells of myelinated fibers containing numerous M. leprae were seen without any obvious ill effects to the myelin (10). Also, in a recent study of M. leprae effects on Schwann cells in vitro, no demyelination was observed after one month (5). It is possible that breakdown of myelin, dependent on contact by M. leprae, may occur, but it is so minimal that it is not likely to be the main cause of leprosy neuropathy.
REFERENCES
1. Binford, C.H. Comprehensive program for inoculation of human leprosy into laboratory animals. Public Health Rep. 71(1956)995-996.
2. Brand, P.W. Temperature variation and leprosy deformity. Int. J. Lepr. 27(1959)1-7.
3. Chehl, S., Ruby, J., Job, C.K. and Hastings, R.C. The growth of M. leprae in nude mice. Lepr. Rev. 54(1983)283-304.
4. Colston, M.J. and Hilson, G.R.F. Growth of M. leprae and M. marinum in congenitally athymic mice. Nature (London). 262(1976)399-401.
5. Hagge, D.A., Robinson, S.O., Scollard, D.M., McCormick, G. and Williams, D.L. A new model for studying the effects of Mycobacterium leprae on Schwann Cells and neuron interactions. J. Infect. Dis. Other Mycobact. Dis. 186(2002)1283-1296.
6. Job, C.K. Pathology of peripheral nerve lesion in lepromatous leprosy. A light and electron microscopic study. Int. J. Lepr. 39(1971)251-268.
7. Job, C.K. and Chacko, C.J.G. A modification of Fite's stain for demonstration of M. leprae in tissue sections. Indian J. Lepr. 58(1986)17-18.
8. Job, C.K., Sanchez, R.M. and Hastings, R.C. Manifestions of experimental leprosy in the armadillo. Am. J. Trop. Med. Hyg. 34(1985)151-161.
9. Rambukkana, A., Zanazzi, A., Tapinos, N. and Salzer, J.L. Contact dependent demyelination by M. leprae in the absence of immune cells. Science. 296(2003)927
10. Scollard, D.M., Lathrop, G.W. and Truman, R.W. Infection of distal peripheral nerves by M. leprae in infected armadillos: An experimental model of nerve involvement in leprosy. Int. J. Lepr. Other Mycobact. Dis. 64(1996)146-151.
11. Scollard, D.M., McCormick, G. and Allen, J.L. Localization of M. leprae to endothelial cells of epineurial and perineurial blood vessels and lymphatics. Am. J. Pathol. 154(1999)1611-1620.
12. Shepard, C.C. The experimental disease that follows the infection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112(1960)445-456.
13. Shetty, V.P. and Antia, N.H. Light and ultrastructure study of sciatic nerve lesions induced by intraneural injection of viable M. leprae in normal and immunosuppressed Swiss white mice. Int. J. Lepr. Other Mycobact. Dis. 70(2002)25-33.
14. Truman, R.W. and Krahenbuhl, J.L. Viable M. leprae as a research reagent. Int. J. Lepr. Other Mycobact. Dis. 69(2001)1-12.
1. M.D., Ph.D., Visiting Scientist; Laboratory Research Branch, National Hansen's Disease Programs at L.S.U.-S.V.M., Skip Bertman Drive, Baton Rouge, Louisiana, U.S.A.
2. B.S., Medical Technologist; Laboratory Research Branch, National Hansen's Disease Programs at L.S.U.-S.V.M., Skip Bertman Drive, Baton Rouge, Louisiana, U.S.A.
3. M.D., Ph.D., Chief, Pathology Research Department; Laboratory Research Branch, National Hansen's Disease Programs at L.S.U.-S.V.M., Skip Bertman Drive, Baton Rouge, Louisiana, U.S.A.
4. Ph.D., Chief, Microbiology Research Department, Laboratory Research Branch, National Hansen's Disease Programs at L.S.U.-S.V.M., Skip Bertman Drive, Baton Rouge, Louisiana, U.S.A.
1 Wayne M. Meyers, M.D., Ph.D., kindly served as Editor in regard to the submission, review, revision, and acceptance of this manuscript.
Reprint requests to: Dr. C. K. Job, St. Thomas Hospital and Leprosy Center, Chettupattu-606 801, Tamil Nadu, India.
Received for publication on 27 February 2003.
Accepted for publication on 13 May 2003.