- Volume 71 , Number 2
- Page: 106–12
Cured tuberculoid patients have a greater life expectancy than cured lepromatous patients in Japan
ABSTRACT
Leprosy patients lack specific cellular immunity against Mycobacterium leprae, but other immunological functions are thought to be preserved. However, in a leprosy sanatorium in South Japan between 1982 and 2000, we found that the average age at death of cured lepromatous leprosy patients was about 5 yrs younger than that of cured tuberculoid patients; [male/lepromatous, 76.0 ± 10.0 yrs old vs. male/tuberculoid, 79.7 ± 9.4 yrs old, p = 0.026], and [female/lepromatous, 78.0 ± 10.5 vs. female/tuberculoid, 85.3 ± 9.8, p = 0.0001]. This trend was also observed in autopsy records of two other leprosy sanatoria in Japan. In a prospective study based on their age in 1982, among females in the age group between 60 and 69, lepromatous patients (75.3 ± 6.0 yrs) died earlier than tuberculoid patients (81.0 ± 5.1 yrs) (p <0.01). These findings suggest that lepromatous patients have higher risk of death even in a post-chemotherapy era.RÉSUMÉ
Les patients souffrant de la lèpre sont démunis d'une immunité à médiation cellulaire spécifiquement contre Mycobacterium leprae, alors que le reste des fonctions immunologiques sont considérées comme préservées. Cependant, nous avons observé que, entre 1982 et 2000, au sein d'un sanatorium de lèpre du Japon méridional, l'âge moyen de décès des patients lépromateux guéris était d'environ 5 années de moins que celui de patients tuberculoïdes guéris ; (patients masculins lépromateux, 76,0 ± 10,0 ans versus patients masculins tuberculoïdes 79,7 ± 9,4 ans, p = 0,026) et (patientes lépromateuses, 78,0 ± 10,5 versus patientes tuberculoïdes 85,3 ± 9,8 ans, p = 0,0001). Cette tendance était aussi présente à l'examen de comptes rendus d'autopsie de deux autres sanatoriums japonais. Dans une étude prospective basée sur des patientes âgées de 60 à 69 ans en 1982, les patientes lépromateuses ont décédé plus tôt (75,3 ± 6,0 ans) que les patientes tuberculoïdes (81,0 ± 5,1 ans) (p <0,01). Ces données suggèrent que les patients lépromateux présentent un plus grand risque de décès, et ceci même à une période qui suit la chimiothérapie.RESUMEN
Los pacientes con lepra carecen de inmunidad celular específica contra Mycobacterium leprae pero otras funciones inmunológicas se mantienen preservadas. Sin embargo, en un estudio realizado en un sanatorio para lepra en el sur de Japón entre 1982 y 2000, encontramos que la edad promedio de muerte de los pacientes con lepra lepromatosa curada fue aproximadamente 5 años menor que la de los pacientes con lepra tuberculoide curada [hombres lepromatosos, 76.0 ± 10 años vs hombres tuberculoides, 79.7 ± 9.4 años, p = 0.026], y [mujeres lepromatosas, 78.0 ± 10.5 vs mujeres tuberculoides, 85.3 ± 9.8, p = 0.0001]. Esta tendencia también se observó en los registros de autopsia en otros dos sanatorios de la lepra en Japón. En otro estudio prospectivo realizado en mujeres de 60 a 69 años en 1982, se encontró que las pacientes con lepra lepromatosa (75.3 ± 6.0 años) murieron más temprana-mente que las pacientes con lepra tuberculoide de la misma edad (81.0 ± 5.1 año) (p <0.01). Estos resultados sugieren que los pacientes lepromatosos tienen mayor riesgo de muerte aun en la era de la post-quimioterapia.In Japan, after the introduction of diaphenylsulfone (dapsone) monotherapy in 1940's and WHO/MDT regimens in 1980's (1), most leprosy patients were clinically cured. However, many of them have sequelae, such as deformities of extremities or face and/or blindness (2), and most of them are still living in Hansen's disease hospitals/sanatoria. Patients having active lesions are extremely rare today.
Leprosy patients show low immunological response to eliminate Mycobacterium leprae, but other immunological responses are considered to be well preserved. Ridley and Jopling classified leprosy into five types (3). Lepromatous patients present Th2 dominant response, and have many bacterial loads, many skin eruptions, and wide damage of peripheral nerves in the skin. Tuberculoid patients show Th1 dominant T cell response and have relatively stronger protective immunity to M. leprae (4), and have less skin eruptions and limited areas of nerve damage. Leprosy is basically a non-lethal disease in both disease types; however, while analyzing the causes of death in a leprosy sanatorium (Hoshizuka-Keiaien), we found that the average age at death is younger in cured lepromatous patients than that of cured tuberculoid patients. In this study, we tried to reveal the reason for this difference.
MATERIALS AND METHODS
Until the abolition of the Leprosy Segregation Act in 1996, many leprosy patients were obliged to stay in national sanatoria in Japan, but several patients were allowed to be discharged during that time. Medical records of inpatients of the National Sanatorium Hoshizuka-Keiaien in Kanoya, Kagoshima, located in the southern part of Japan, were reviewed and a basic computer database was established in 1982. In 1982, there were 751 patients. They were followed up until 2000, and their death records were analyzed. Age at 1982, age at admission, age at death (between 1982 and 2002), gender, type of the disease, blindness, and Mitsuda's skin reaction were analyzed. In this study, types of leprosy were simply classified as lepromatous or tuberculoid, the former includes polar lepromatous (LL), borderline lepromatous (BL), and mid-borderline lepromatous (BB) while the latter includes borderline tuberculoid (BT) and polar tuberculoid (TT), according to the Ridley and Jopling classification (1). A small number of cases were classified as borderline type not otherwise specified.
A summary of autopsy results has been published in Japan every year (5). The published data of two other national leprosy sanatoria Tama-Zenshouen, Tokyo and Oku Komyoen, Okayama between 1982-2000 were compared with the autopsy results of Hoshizuka-Keiaien.
RESULTS
Profile of patients in Hoshizuka-Keiaien in 1982. In 1982, there were 518 patients with lepromatous leprosy (LL, BL and BB; male, 338; female, 180), 223 patients with tuberculoid leprosy (BT and TT; male, 91; female, 132), and 10 patients of borderline not otherwise specified (male, 4; female, 5) in the National Sanatorium Hoshizuka-Keiaien. Among them, 205 lepromatous patients (male, 146; female, 59), 107 tuberculoid patients (male, 9; female, 58) and 1 patient of borderline not otherwise specified (female) died before the end of 2000. In total, among 751 patients, 311 patients (41.4%) died in 18 yrs. Cases of borderline not otherwise specified are excluded from the following analyses.
Age distribution (in yrs) of lepromatous and tuberculoid patients for both genders in 1982 was analyzed. As shown in Table 1 , male patients with tuberculoid leprosy (63.3 ± 9.9) were 3.7 yrs older than male patients with lepromatous leprosy (59.6 ± 10.3) (p <0.01 by Mann-Whitney's U-test). Also, female patients with tuberculoid leprosy (64.7 ± 12.7) were 5.8 yrs older than female patients with lepromatous leprosy (59.6 ± 10.3) (p <0.005 by Mann-Whitney's U-test).
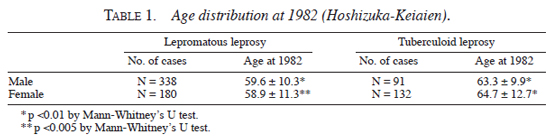
Data of age at the onset of leprosy were difficult to obtain in many cases, thus their age at the first admission to leprosy sanatorium was analyzed. Among the 741 cases in 1982, data of 532 cases (71.8%) were available. As shown in Table 2 , male patients with tuberculoid leprosy (29.3 ± 11.6) admitted almost at the same age as male patients with lepromatous leprosy (29.6 ± 12.0) (not statistically significant). Also, female patients with tuberculoid leprosy (29.2 ± 14.3) admitted almost at the same age as female patients with lepromatous leprosy (26.9 ± 12.7) (not statistically significant).

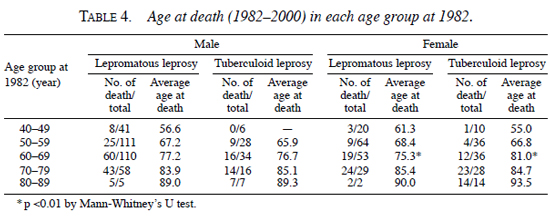
Age at death in Hoshizuka-Keiaien between 1982 and 2000. As shown in Table 3 , the age (yrs) of the patients at their deaths were male/lepromatous, 76.0 ± 10.0; male/tuberculoid, 79.7 ± 9.4; female/lepromatous, 78.0 ± 10.5; female/tuberculoid, 85.3 ± 9.8. Their average age of death was 3.9 yrs higher in male/tuberculoid than in male/lepromatous patients (p = 0.026), and it was 7.3 yrs higher in female/tuberculoid than in female/lepromatous patients (p = 0.0001) by Student's t test. In addition, the age of death in female/lepromatous patients was 5.6 yrs higher than in male/lepromatous patients (p = 0.003).
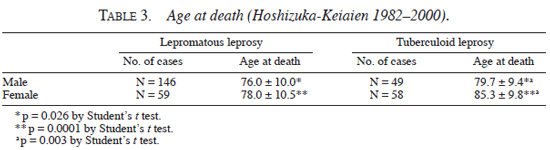
The age at death based on their age at 1982 was analyzed, which is shown in Table 4. In most age groups of both genders, patients with lepromatous and tuberculoid leprosy died almost at the same age. However, female lepromatous patients in the age group between 60 and 69 died (75.3 ± 6.0) 5.7 yrs earlier than female tuberculoid patients (81.0 ± 5.1) (p <0.01 by Mann-Whitney's U test).
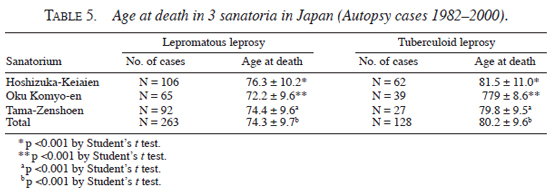
Age of death in three sanatoria-analysis of autopsy record. An autopsy was performed when permission was obtained, irrespective of the patients' age or cause of death. At Hoshizuka Keiaien, a total of 168 cases of autopsy were done from 1982 to 2000. Lepromatous patients were 106 (male, 75; female, 31) and tuberculoid patients were 62 (male, 30; female, 32). At Oku Komyoen in Okayama, 104 cases of autopsy were done in the same period. Lepromatous patients were 65 (male, 54; female, 11) and tuberculoid patients were 39 (male, 22; female, 17). At Tama-Zenshoen in Tokyo, 119 autopsies were done in the same period. Lepromatous patients were 92 (male, 62; female, 30) and tuberculoid patients were 27 (male, 13; female, 14). As shown in Table 5 , the average age at death was about 5 yrs higher in tuberculoid patients than that in lepromatous patients (p <0.001 by t test), despite the fact that average age at death was different among the three sanatoria (e.g., lepromatous in Hoshizuka-Keiaien, 76.3 yrs; lepromatous in Oku Komyoen, 72.2 yrs).
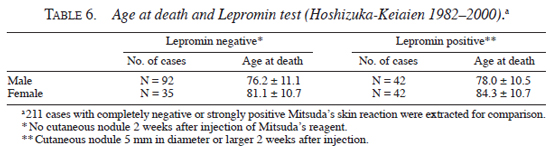
Analysis of other risk factors. In order to find out risk factors of death other than leprosy types in Hoshizuka-Keiaien, several studies were performed. First, status of lepromin test (Mitsuda's skin reaction (6)) and age at death were compared for both genders. Lepromin test was performed at various periods and recorded as nodule size (large x small diameter in mm), from which average diameter was calculated. As shown in Table 6 , the average age of death of male patients with strong positive skin reaction (nodule size 5 mm) was 78.0 ± 10.5 yrs, and that of completely negative skin reaction was 76.2 ± 11.1 yrs. Average age of death of female patients with strong positive skin reaction was 84.3 ± 10.7 yrs, and that of completely negative skin reaction was 81.1 ± 10.7 yrs. The age at death of patients with strong lepromin tests tended to be higher than that of those with negative lepromin tests, but it was not statistically significant.
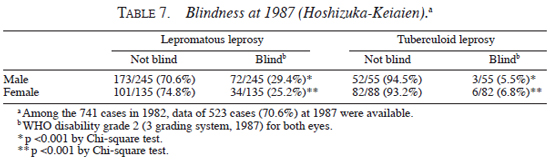
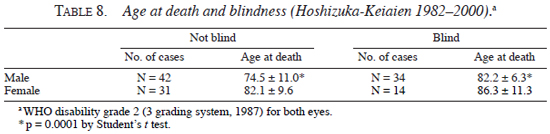
Second, blindness and the age at death were analyzed. As shown in Table 7 , the frequency of blindness was significantly lower in patients with tuberculoid leprosy than in patients with lepromatous leprosy, both for male (tuberculoid, 5.5%; lepromatous, 29.4%, p <0.001 by Chi-square test) and female (tuberculolid, 6.8%; lepromatous, 25.2%, p <0.001 by Chi-square test). On the other hand, as shown in Table 8 , the average age at death of male blind patients (82.2 ± 6.3 yrs) was higher than that of male non-blind patients (74.5 ± 11.0 yrs) (p <0.0001 by Student's t test), and average age at death of female blind patients (86.3 ± 11.3 yrs) showed higher tendency than that of female non-blind patients (82.1 ± 9.6 yrs) (statistically not significant).
DISCUSSION
Furuta, et al. reported an analysis of autopsy cases in a Japanese leprosy sanatorium Oku Komyoen (7). Their study focused on the malignant diseases, and they did not mention the difference of age at death between lepromatous and tuberculoid cases.
Before starting this study, we did not expect that cured tuberculoid leprosy patients live longer than lepromatous patients. However, the average age at the time of death between 1982 and 2000 was approximately 5 yrs (male, 3.7 yrs; female, 7.3 yrs) older in tuberculoid patients than lepromatous patients in Hoshizuka-Keiaien. This tendency was also observed in the other two leprosy sanatoria's autopsy records of the same period, including Oku-Komyoen where Furuta, et al. (7) studied. The average age at death per se was different among the three sanatoria, but overall the age at death was approximately 5 yrs older in tuberculoid patients than in lepromatous patients.
For both genders, the older age distribution of tuberculoid patients at the beginning of this retrospective cohort study in 1982 was the most influential factor for the older age at death in the tuberculoid group. Here, we postulate three possibilities as the cause of such differences and discuss the hypothesis for each.
First, the difference of age distribution may be due to the younger onset of lepromatous leprosy than that of tuberculoid leprosy. In Japan, Ozaki and Tomoda (8) and Ishii, et al. (9) reported surveys of newly diagnosed cases, but they did not analyze the age distribution of the two types of the disease. In an epidemiological study performed in Andhra Pradesh, India, de Vries and Perry (10) reported that the peak of leprosy prevalence and incidence for both tuberculoid and lepromatous cases was in the age group 35-44 yrs, and both males and females showed similar age distribution. In this study, we could not obtain reliable data concerning the age at the onset of the disease, but the age at admission was available in 71.4% of the patients, and they first entered the sanatorium in late 1920's in both disease types and gender groups. Thus, this possibility seems unlikely.
Second, we suspected that some socio-economic factors have biased the age distribution of patients living in leprosy sanatoria. Even if the original age distribution between tuberculoid and lepromatous patients were the same in leprosy sanatoria before 1982, young tuberculoid patients having only mild deformities could be discharged and make their own living more easily than young lepromatous patients. This should result in a decrease of young tuberculoid patients and a shift to older age. If this hypothesis is correct, the tuberculoid group should show a narrower range of age distribution than that of the lepromatous group. However, as shown in Table 1 , the standard deviation of age was almost identical in both male (tuberculoid, 9.9; lepromatous, 10.3) and female (tuberculoid, 12.7; lepromatous, 11.3) groups. Thus, we could not prove this hypothesis. On the other hand, immunological parameters, other than clinical classification of lepromatous and tuberculoid leprosy, were looked for. For this purpose, lepromin skin reaction was selected, which is still an indicator of host's cellular immunity against M. leprae. The Lepromin test is usually graded as negative (0 mm), ± (0.1-2.9 mm), positive (3.0-4.9 mm) and strongly positive (5 mm or larger). Negative and strongly positive groups were compared for their age at death, and the latter group showed a tendency of higher age (1.8 yrs for male and 3.2 yrs for female), but it was not statistically significant. We do not think that our clinical diagnosis of lepromatous and tuberculoid types was inadequate, instead, lepromin test provided us limited information for the integrated clinical classification of leprosy.
Third, the difference of age distribution may be the result of a higher death rate in lepromatous patients. Leprosy itself is basically a non-lethal disease, but acute laryngeal obstruction in leprosy reaction could be a cause of death, especially before the introduction of dapsone therapy (11). In the observation period between 1982 and 2000, a small number of cases with erythema nodusum leprosum (ENL) were encountered in our study, but they were well controlled and there was no acute renal failure or laryngeal edema due to ENL. By the 6 yr follow up in South India between 1962 and 1968, Noordeen demonstrated that lepromatous cases in the field had a mortality of about 3.5 times that of the general population and twice that of non-lepromatous cases (12). But he also found that lepromatous cases admitted into the sanatorium had a death rate very close to that of the general population. Similarly, Oleinick demonstrated that in the U.S. Public Health Service Leprosarium in Carville between 1939 and 1963, the mortality risk of patients with both types of leprosy was normal (13). Because of the good nursing care in Japanese leprosaria, at least after 1982, malnutrition due to deformity was not observed. Male blind patients lived 7.7 yrs longer than male non-blind patients. Therefore, the possibility of poor nutritional status due to blindness was not observed. However, a shorter life expectancy of females with lepromatous leprosy than that of those with tuberculoid leprosy in the age group 60-69 in 1982 was demonstrated in our present study. Lepromatous patients have more deformities of the face, nose, or mouth, which may increase respiratory tract infection. Also, hand and foot ulcers can cause abscess and osteomyelitis followed by sepsis, but sepsis due to planter ulcers was not the cause of death in this period. Although as much as 25% of lepromatous patients were skin smear positive in Hoshizuka-Keiaien because of inadequate control in 1970's, it became 3% in 1991 (14) and almost zero in 2000. Dementia due to reduced activity related to deformity would cause younger death in lepromatous patients (15). At the moment, it seems reasonable to suggest that lepromatous patients have a higher risk of death even in the post-chemotherapy era.
In this study, we could not clarify whether different immune status between lepromatous and tuberculoid patients directly affected their life span. Furuta, et al. (7) showed that malignant tumors were found in 31.5% of lepromatous cases and in 38% of tuberculoid cases in Oku Komyoen. On the contrary, Tokudome, et al. (16) demonstrated that deaths from total malignant neoplasms were higher than expected among patients with lepromatous leprosy for both sexes, whereas they were lower than expected among tuberculoid patients in Kikuchi Keifuen. Further detailed analysis of malignant neoplasm in both types of the disease in Hoshizuka-Keiaien is necessary in order to answer this question.
After the introduction of the WHO/MDT regimen, leprosy has become a curable disease. But if life expectancy is different between the disease types in cured leprosy patients like Japan, it should be carefully followed up after the release from control.
Acknowledgments. The authors appreciate Dr. Kentaro Hatano, 0ku Komyoen and Dr. Mikihisa Yajima, Tama-Zenshoen for allowing us to use the published autopsy records. The authors thank Ms. Taki Hatanaka for excellent technical help. This work was supported by a Health Research Grant on Emerging and Re-emerging Infectious Diseases, Ministry of Health, Labor and Welfare, Government of Japan. Also, this work was partly supported by the U.S.-Japan Cooperative Medical Science Program.
REFERENCES
1. de Vries, J.L. and Perry, B.H. Leprosy case detection rates by age, sex, and polar type under leprosy control conditions. Am. J. Epidemiol. 121(1985)403-413.
2. Furuta, M., Obara, A., Ishida, Y., Harada, N. and Ozaki, M. Leprosy and malignancy: autopsy findings of 252 leprosy patients. Int. J. Lepr. 58(1990)697-703.
3. Goto, M., Kimura, T., Hagio, S., Ueda, K., Kitajima, S., Tokunaga, H. and Sato, E. Neuropathological analysis of dementia in a Japanese leprosarium. Dementia. 6(1995)157-161.
4. Goto, M., Kitajima, S. and Imaizumi, M. Number of leprosy patients in Japan-a survey in 1995. Int. J. Lepr. 66(1998)80A
5. Goto, M., Suzuki, M., Kitajima, S. and Imaizumi, M. Changes and present status of a Japanese national leprosarium-analysis of smear positive rate and relapse in Hoshizuka-Keiaien between 1972-1991. Jpn. J. Lepr. 62(1993)1-12.
6. Hayashi, F. Mitsuda's skin reaction in leprosy. Int. J. Lepr. 1(1933)31-38.
7. Ishii, N., Onoda, M., Sugita, Y., Tomoda, M. and Ozaki, M. Survey of newly diagnosed leprosy patients in native and foreign residents of Japan. Int. J. Lepr. 68(2000)172-176.
8. The Japanese Society of Pathology. Annual Report of the pathological autopsy cases in Japan. Vol. 25-43. Japanese Society of Pathology, Tokyo. (1982-2001). (in Japanese.).
9. Noordeen, S.K. Mortality in leprosy. Ind. J. Med. Res. 60(1972)439-445.
10. Oleinick, A. Survival among leprosy patients with special consideration of cancer as a cause of death. Int. J. Lepr. 36(1968)318-327.
11. Ozaki, M. and Tomoda, M. Decrease of newly registered leprosy patients in Japan-epidemiological study of leprosy as non-endemic disease. Jpn. J. Dermatol. 103(1993)1867-1876.
12. Pfaltzgraff, R.E. and Ramu, G. Clinical leprosy. In: Leprosy. 2nd edn. Edinburgh: Churchill Livingstone, 1994, pp. 237-287.
13. Ridley, D.S. and Jopling, W.H. Classification of leprosy according to immunity-a five group system. Int. J. Lepr. 54(1966)255-273.
14. Tokudome, S., Kono, S., Ikeda, M., Kuratsune, M. and Kumamaru, S. Cancer and other causes of death among leprosy patients. J. Natl. Cancer Inst. 67(1981)285-289.
15. WHO. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
16. Yamamura, M. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 254(1991)277-279.
1. M.D., Ph.D., Associate Professor; Department of Pathology, Kagoshima University Faculty of Medicine, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan.
2. M.D., Ph.D.; Department of Pathology, Kagoshima University Faculty of Medicine, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan.
3. M.D., Ph.D., Director Professor; Department of Pathology, Kagoshima University Faculty of Medicine, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan.
4. M.D., Ph.D., Associate Professor, Division of Surgical Pathology, Kagoshima University Hospital.
5. M.D., Division of Research and Examination; National Hansen.s Disease Hospital Hoshizuka-Keiaien, Kagoshima, Japan.
6. Director General; National Hansen.s Disease Hospital Hoshizuka-Keiaien, Kagoshima, Japan.
Reprint requests to: Masamichi Goto, Department of Pathology, Kagoshima University Faculty of Medicine, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan; E-mail: masagoto@m2.fufm.kagoshima-u.ac.jp
Received for publication on 24 September 2002.
Accepted for publication on 5 March 2003.