- Volume 65 , Number 2
- Page: 157–65
Study of brain stem auditory-evoked potentials (BAEPs) and visual-evoked potentials (VEPs) in leprosy
ABSTRACT
A study of brain stem auditory-evoked potentials (BAEPs) and visual-evoked potentials (VEPs) was done on 25 newly diagnosed patients with leprosy whose diagnosis was confirmed by skin biopsy. The results were compared with 25 age- and sex-matched healthy controls. In BAEPs the important observations were the prolonged latency of wave V in 13 (52%), delayed interpeak latency (IPL) of wave I-III in 5 (20%) cases, of wave III-V in 12/25 (40%) , suggesting a conduction abnormality of the VIII cranial nerve in its peripheral part, in its nucleus and in its connection in the brain stem. In VEPs, a delayed peak latency of major positive potential (PI00 ) was seen in 20 cases (80%; 11/13, 84.6% TT; 7/10, 70 % LL; 2/2, 100% BL), suggestive of subclinical optic nerve involvement. The BAEPs and VEPs were both abnormal in 10 cases (40%; 3/13, 23 % TT; 5/10, 50 % LL; 2/2, 1007C BL). Conduction abnormalities of the central nervous system (CNS) were observed more frequently in lepromatous leprosy, as in other forms of peripheral neuropathy such as hereditary motor sensory neuropathy type I (HMSN I). There is a fair possibility of similar multiple demyelinating lesions in the CNS also, as is seen in leprous peripheral neuropathy. This hypothesis requires further strengthening by an extensive study of multimodality evoked potentials with magnetic resonance imaging in the patients. Histopathological and immunofluorescent studies of autopsy material of the brain can also contribute significantly to solve the dilemma.RÉSUMÉ
Une étude des potentiels evoques cérébraux auditifs (PECA) et des potentiels evoques visuels (PEV) a été réalisée chez, 25 malades de la lèpre nouvellement diagnostiqués pour lesquels le diagnostic de lèpre était confirmé par biopsie cutanée. Les résultats ont été comparés avec ceux de 25 témoins en bonne santé appariés pour l'âge et le sexe. Lu ce qui concerne les PECA, les observations importantes ont été la latence prolongée de l'onde V chez 13 patients (52%), la latence inter-pics retardée de l'onde I-III chez. 5 patients (20%), de l'onde III-V chez 12/25 patients (40%), suggérant une anomalie de conduction du huitième nerf crânien dans sa partie périphérique, dans son noyau et dans sa connection dans le cerveau. En ce qui concerne les PEV, un pic de latence retardé de potentiel positif majeur (PI00) a élé vu dans 20 cas (80%; 11/13, 86% TT; 7/10, 70% LL; 2/2, 100% ML), suggérant une implication subclinique du nerf optique. Les PECA et PEV étaient tous deux anormaux dans 10 cas (40%; 3/13, 23% TT; 5/10, 50% LL; 2/2, 100% BL). Des anomalies de conduction du système nerveux central (SNC) ont été observées plus fréquemment dans la lèpre lépromateuse, comme dans d'autres formes de neuropathic périphérique telles que la neuropathic sensori-motrice héréditaire de type 1 (NSMII I ). Il y a une bonne possibilité de multiples lésions démyélinisantes similaires dans le SNC également, comme cela est vu dans la neuropathic lépreuse périphérique. Cette hypothèse demande à être plus étayée par une étude extensive de la multimodalité des potentiels évoqués avec résonance magnétique chez les patients. Des études histopathologiques et d'immunolluorescence sur du matériel d'autopsie du cerveau peuvent aussi contribuer signilicativement à résoudre le dilemme.RESUMEN
Se estudiaron los potenciales auditivos evocados del tronco cerebral (PAECs) y los potenciales visuales evocados (PVEs) en 25 pacientes con lepra confirmada por biopsia de piel. Los resultados se compararon con los encontrados en 25 controles sanos de edad y sexo similares. En los estudios sobre los PAECs las observaciones importantes fueron la latencia prolongada de la onda V en 13 pacientes (52%), una latencia retardada entre los picos de las ondas 1-I1I en 5 casos (20%) y de las ondas I1I-V en 12 de 24 pacientes (40%). Los resultados sugieren una anormalidad en la capacidad de conducción del nervio cranial VIII en su parte periférica, en su núcleo y en su conexión con el tronco cerebral. En los PVEs se observó una latencia retardada de los picos con mayor potencial positivo (P100) en 20 casos (80%): 11/13 TT (84.6%), 7/10 LL (70%) y 2/2 BL (100%), sugerente de una afección subclinics del nervio óptico. Tanto los PAECs como los VEPs fueron anormales en 10 casos (40%): 3/13 TT (23%), 5/10 LL (50%) y 2/2 BL (100%). Dado que las anormalidades en conducción del sistema nervioso central (SNC) fueron tan frecuentes en la lepra lepromatosa como en otras formas de neuropatía periférica (como la neuropatía sensorial motora hereditaria, HMSN 1), existe la posibilidad de que también en el SNC haya múltiples lesiones desmielinizantes, como se ve en la neuropatía leprosa periférica. Para confirmar esta hipótesis se requiere un estudio más extenso sobre la multimodalidad de los potenciales evocados en los pacientes usando imágenes de resonancia magnética. Los estudios histopatológicos y de inmunofluorescencia en el material de autopsia del cerebro también puede contribuir a resolver el dilema.Leprosy is a systemic infectious disease which has a predilection for involvement of the skin, peripheral nerves and the mucosa of the upper respiratory tract. It is endemic in tropical countries, and India has the highest incidence and prevalence rate. It is the commonest disease of peripheral nerves in man. A cardinal sign is sensory loss which always precedes paralysis in all types of leprosy (31). Clinically, motor involvement is less common but patho-physiologically it is almost equal to the sensory involvement (l3,32). Nerve involvement occurs prior to any clinical manifestation (1) and any nerve in the body can be affected. Nerve involvement in leprosy is segmental demyelinating in nature initially, followed by axonal degeneration (1,5). Among the cranial nerves commonly affected are V, VII, statoacoustic (4,10,18) and optic (11,34,37). The possibility of a relationship between hearing loss and leprosy was not thought of until Latif (19) reported VIII nerve involvement in up to 25% of his patients and Schilling and lstre (31) observed that it was not due to middle ear or eustachian tube pathology. El Arini, et al. (10) reported gradual and progressive hearing loss in 11% of the patients in a study of 101 leprosy patients; a few of them were also having vestibular dysfunction. Singh, et al. (33) reported evidence of both perceptive and conductive dysfunction, and found hearing loss in 52% and vestibular dysfunction in 7.2% of their patients. Conductive hearing deafness was prominent in a survey by Srinivasan (35). Recently, Awasthi, et al. (2) have observed that only the cochlea was involved and the vestibule was spared. DeCandia and Mariana (6) reported specific evidence of cochlear and acoustic nerve damage by audiogram.
Brain stem auditory-evoked potentials (BAEPs) are used to determine the abnormality of conduction from the auditory nerve to the inferior colliculus. It is a very useful procedure for evaluating the integrity of the VIII cranial nerve, nucleus and its connections in the brain stem and for detecting the lesion at a subclinical stage. Visual evoked potentials (VEPs) are very useful for detecting a lesion in the optic nerve, and their results are comparable to magnetic resonance imaging (MRI). This study was done for assessment of subclinical abnormality in the optic and stato-acoustic nerves by VEPs and BAEPs in this leprosy-endemic region.
MATERIALS AND METHODS
The present study was conducted on 25 newly diagnosed patients (21 males and 4 females; ages ranging from 15 to 55 years) with different types of leprosy. The patients were selected from a leprosy clinic conducted by the Skin and Sexually Transmitted Disease Department of S.P. Medical College and Associated Group of Hospitals, Bikaner, India.
The diagnostic criteria for leprosy were those mentioned by Dharmendra (7): a) loss of sensation in patches, b) thick and/or tender nerves, c) routine slit and smear examination of skin for the demonstration of acid-fast bacilli (AFB). One or more than one criteria were fulfilled before a diagnosis ofleprosy was made and confirmation was done by skin biopsy.
A detailed clinical and neurological examination was carried out in each case, and the cases were classified into different types of leprosy according to the classification given by Ridley and Jopling (28) which is recommended by the Indian Association of Leprologists (7). The diagnosis was confirmed by skin biopsy. The other investigations included hemoglobin, total and differential leukocyte counts, erythrocyte sedimentation rate, urinalysis and liver function tests. Patients with any other associated disease were not included in the study. A detailed drug history was also taken to exclude the presence of toxic neuropathy.
After classifying the patients into different groups, we included 13 patients with tuberculoid (TT), 10 patients with lepromatous (LL) and 2 patients with borderline lepromatous (BL) leprosy; 25 healthy persons (18 males and 7 females; ages ranging from 15 to 55 years) without any organic illness or history of drug treatment served ascontrols. The patients and controls were ex-amined thoroughly for visual acuity, color blindness, and other eye abnormalities along with detailed ear examinations and hearing tests. Patients and controls having colorblindness, eyelid drooping, refractive error,corneal and lens opacity or with any ear disease were excluded from the study.
BAEPs and VEPs were recorded for all of the patients and control subjects in a shielded, partially sound-proof, dimly lighted room.The computerized machine used was MULTIBASIS (OTE-BIOMEDICA) which includes console, green fluorescent screen with functional keys and floppy disc driver,connected to a dot matrix printer.
BAEPs. BAEPs were recorded between the vertex and the ipsilateral mastoid process using monaural alternate click stimuli of 0.1 millisecond duration with a stimulus intensity of 60 dB above the auditory threshold. The contralateral ear was masked with white noise of +10 dB (50 dB below the click stimulus level). The click was delivered at a regular rate of 10 per second.
The right and left ears were stimulated independently. At least two separate trials, each consisting of 2000 stimuli, were amplified (5 X 105) and averaged with band pass filters at 100 Hz and 3000 Hz. Data were analyzed in detail with digital latency and amplitude cursors. Different parameters used for the analysis of the results were: peak latencies of wave I, wave II, wave III and wave V components and interpeak latencies (IPLs) of wave I -111, wave 111 - V, wave I-V and amplitude ratio of wave V/I.
VEPs. Pattern reversal VEPs were recorded between the Oz (reference point) and the contralateral mastoid (international 10-20 systems). A black-and-white checkerboard pattern subtending a visual field of 15º X 18º with an individual check size of 30' was displayed on the television monitor and reversed once per second. The subject was asked to sit 60 cm away from the screen and focus on a small fixation point in the center of the pattern. Illumination of the entire pattern was kept fixed. Responses were recorded by a needle electrode placed on the scalp in midline 3 cm above the inion (point of the external occipital protuberance); a contralateral mastoid electrode was used as reference. Each eye was tested separately while the other eye was kept covered. The responses to 100 stimuli were averaged and at least two stimulation series were recorded. The major positive potential (PI00) latency and amplitude of the VEPs' peaks were measured for analysis of the results.
All data were statistically evaluated and different comparisons were made by Student's t test. Values were considered abnormal only when they were beyond ± 2.5 S.D. of the control mean values.
RESULTS
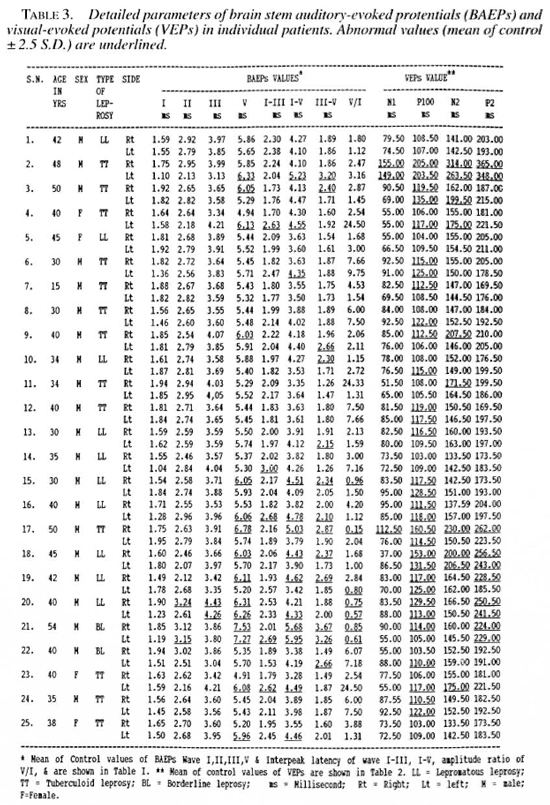
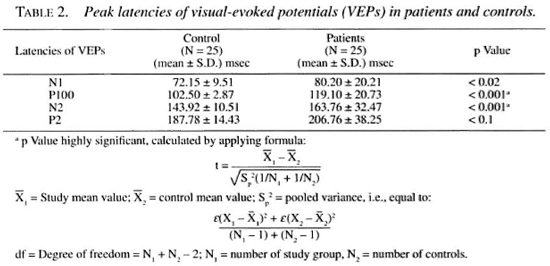
The results of the BAEPs shown in Table 1 revealed increased peak latency of wave I in none, wave II in 2 case (4% LL, 4% BL), wave III in 1 case (4%, LL), and wave V in 13 cases (52%; 7 TT, 5 LL, 1 BL); interpeak latencies (IPLs) were prolonged for wave I-III in 5 cases (20%; 2 TT, 2 LL, 1 BL), for wave III-V in 12 cases (48%; 6 LL, 4 TT, 2 BL). The wave V/I amplitude ratio was less than 1 in 5 cases (20%; 3 LL, 1 TT, 1 BL). No patient had any absent wave. The values of latency of waves I, II, III and the IPLs of wave I-III and the V/I ratio of amplitude were statistically insignificant. The most important abnormalities were the delayed peak latency of wave V and the delayed IPLs of wave III-V and wave I-V.
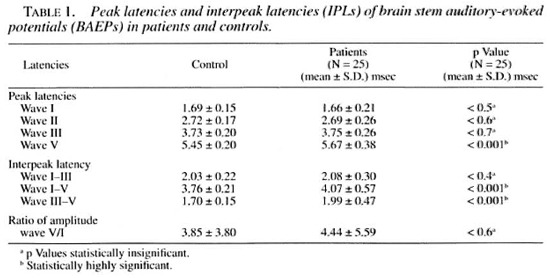
The results of the VEPs as shown in Table 2 demonstrate delayed peak latency of N1 in 2/25 cases (8%; both TT), of major positive potential (PI00) in 20/25 cases (80%; 11 TT, 7 LL, 2 BL), of N2 in 8/25 cases (32%; 7 TT, 1 LL) and of P2 in 6/25 cases (24%; 2 TT, 3 LL, 1 BL). The important observation was the delayed peak latency of P100 in 807c of the cases ( 11 TT, 7 LL, 2 BL).
DISCUSSION
Leprosy is a systemic infective disorder which primarily involves the peripheral nerves in the form of segmental demyelination leading to axonal degeneration in the later period (1,5). Clinical examination reveals evidence of peripheral neuropathy only, but there are various reports indicating involvement of cranial nerves also (4,10,11,18,34,37). Central demyelination has been observed in some of the other primarily peripheral nerve diseases, especially hereditary motor sensory neuropathy (HMSN) type 1 which also produces peripheral neuropathy with segmental demyelination (12,17,30) . Lately there have been a few reports suggesting brain stem demyelination in these disorders (17). This prompted us to investigate the same type of lesion in leprosy along with the subclinical involvement of the optic and stato-acoustic nerves.
BAEP is the latest method which provides information about the integrity of the primary and secondary neural pathway from the cochlea to the temporal lobe cortex. The short latency BAEPs provide up to a series of seven vertex positive waves appearing at the scalp within 10 milliseconds after each acoustic stimulus (15,25,26). These component waves have been labeled with Roman numerals I to VII. The generator sites for these components, based on pathological correlates and on direct recording from the brain stem at the time of operation, suggest the following: waves I and II are generated from the peripheral and intracranial part of the auditory nerve, respectively, while wave III is generated from the cochlear nucleus, wave IV from the superior olive, and wave V from the lateral lemniscus. The generator of waves VI and VII is uncertain, possibly from the inferior colliculus (9). A lesion that affects one of the relay stations or its immediate connections is said to be manifested by lower voltage of the wave or a delay in its appearance, and the absence or reduction in amplitude of subsequent waves. These effects are more pronounced on the side of the stimulated ear than contralaterally, which is difficult to understand since the majority of the cochlear-superior olivarylateral lemniscal-medial geniculate fibers cross to the opposite side. It is also surprising that a severe lesion of one relay station would allow the impulses to continue their ascent and be recordable in the cerebral cortex. The assessment of the brain stem can be achieved through measuring the interpeak conduction times (waves I-III, III-V or I-V) and relative amplitude of wave V to 1 (14). It is important to stress that normal BAEPs may be seen in deafness due to a cortical lesion (16,23,27,41).
In this study at least one abnormal BAEP component was observed in 18/25 patients (72%; 8 TT, 8 LL, 2 BL). The important abnormalities detected were the delayed absolute peak latency of wave V in 13/25 (52%; 7 TT, 5 LL, 1 BL) and the prolonged interpeak latency of wave III-V in 12/25 (48%; 4 TT. 6 LL, 2 BL). A delay in the absolute peak latencies of waves I, II and III was less frequent. The wave V/I amplitude ratio was abnormal (i.e., less than one) in 5/25 (20%; 3 LL, 1 TT, 1 BL). These data suggest involvement of the connecting fibers in the pons and midbrain.
We could not find other studies of BAEPs in patients with leprosy for comparison with our results. However, a similar study is available in a number of other diseases which also affect the peripheral nerves and does not have clinical evidence of brain stem involvement, such as Guillain-Barre syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP) and HMSN. So, we compared our results with the observations of these diseases.
Short latency BAEPs were recorded in 57 patients of HMSN disease (l7). Abnormal BAEPs were observed in almost 50% of the patients. The most frequent finding was prolongation of I-III IPLs detected in 16 (28.1%) patients. Prolongation of wave III-V interpeak latency was detected in 6 (10.5%; 5 with HMSN type I and 1 with HMSN type II). Wave I-V IPLs were prolonged in 12 patients (10 HMSN type I and 2 HMSN type II). The prolongation of various IPLs was more pronounced in HMSN type I than in HMSN type II. The basic process in HMSN type I is demyelination; in HMSN type II it is axonal degeneration. The above observations suggest that central involvement is more common in demyelinating peripheral neuropathies than in axonal degeneration (l7). In leprosy, also, the basic process is demyelination which ultimately leads to axonal degeneration. In HMSN type I, the same results were also reported by other workers (l2,30). The short latency BAEPs in 27 patients with acute GBS led to the conclusion that central BAEP abnormalities in this disease, though rare, may reflect small, clinically unexpressed inflammatory lesions in the brain stem (29). These studies are in favor of the central nervous system (CNS) involvement in the concerned disease, although there is no pathological or clinical corelate to support this conclusion.
BAEPs and MRI studies in six patients with C1DP revealed BAEP abnormalities in 2/6 (33%) patients. These abnormalities were considered as indicative of a brain stem lesion on the affected side (36). In another study, abnormal BAEPs were recorded in 2/8 (25%) patients of C1DP, demonstrating focal demyelination of the extramedullary portion of the auditory nerve in one and delayed III-V IPLs in the other patient.
In our present study delayed IPLs I-111 and 11 I-V and the abnormal V/I amplitude ratio indicate the involvement of the peripheral as well as the central part of the VIII cranial nerve in the brain stem. CNS involvement also has been reported earlier by El Arini, et al. (10).
Since the cochlear nucleus is connected with the cortex of both temporal lobes, hearing is unaffected by a unilateral cerebral lesion. Deafness due to brain stem lesions is observed only rarely, since a massive lesion is required to interrupt both the crossed and uncrossed projection from the cochlear nucleus. It has to be so massive that other neurologic abnormalities due to the lesion make the testing of hearing impossible. On the other hand, a lesion before the cochlear nucleus can cause deafness. So, a demyelinating lesion, which is not unlikely in leprosy before the cochlear nucleus, can cause deafness. Demyelination results from the destruction of Schwann cells by an uncontrolled intracellular proliferation of leprosy bacilli for a prolonged period with minimal interference with cellular functions, provided there are no host immune responses. Eventually, competition for cellular metabolites or pure space (40) occupying effects(24) of the large number of bacilli lead to vacuolation and foamy de generation of the Schwann cells and causes segmental demyelination. Obviously, segmental demyelination is more common in lepromatous leprosy. Ischemic neuropathy also might play a role in causing segmental demyelination in leprosy (3,8). It will be prudent to keep the possibility of these segmental demyelinating lesions responsible for the abnormalities in BAEPs.
VEPs. VEPs are a measure of the electrical activity between the retina and the occipital pole and can be used as a clinical tool to detect abnormalities of the visual system. Miller, et al. (20) reported that VEP is an important method to detect an acute attack of optic neuritis. They compared the detection of acute and old cases of optic neuritis by VEPs and MRI and found VEPs superior in both conditions.
Normally it is a triphasic response, a major positive peak preceded and followed by negative peaks, but assessment of the visual pathway can be done by amplitude and latency of" PI00. It is seen that amplitude has a better correlation with visual acuity. In an acute attack of retrobulbar neuritis, visual acuity is greatly reduced, the amplitude of the pattern response is correspondingly diminished, and the response may be unobtainable at this stage if the acuity is reduced to counting fingers or to the perception of light. In a typical case, this acute stage lasts only for a day or two and is followed by rapid improvement in the visual acuity, often with complete restoration of normal vision within a month. The pattern reversal evoked potential returns to near normal amplitudes in parallel with the recovering vision. The outstanding change in the pattern reversal VEPs is, however, in the latency of the major positivity (PI00) following the attack (l4). In our present study peak latency of PI0 0 was delayed in 20/25 patients (80%; 11 TT, 7 LL, 2 BL). Eleven cases had bilateral delayed peak latency of PI0 0 (6 TT, 5 LL). Since we do not have similar studies in leprosy, we tried to correlate it with similar studies in other peripheral nerve diseases.
Pattern reversal VEPs were studied in a group of 57 patients of HMSN and delayed P100 latency was observed in 16 patients (28.1%); somewhat more often in HMSN I, in 12 of 37 patients (32.4%), than in 4 of 20 HMSN II patients (20%), probably because of more common demyelination in HSMN I (7). VEPs and MRI were studied in 7 patients with CIDP and delayed latencies of PI0 0 were observed in 6 patients; 2 patients also had MRI evidence of CNS involvement. It was suggested that immunomediated damage was responsible for optic nerve involvement in these patients (38).
In the present study, we found delayed peak latency of PI00 in 20/25 (80%) cases of leprosy, suggesting subclinical optic nerve involvement. Clinical involvement of the optic nerve in patients with leprosy also has been reported by earlier workers (21). Van Poole (39) described optic neuritis in 49/206 cases of leprosy.
Assessment of the results of VEPs and BAEPs in leprosy in this study reveals the abnormality of both evoked potentials in 10/25 , suggesting subclinical involvement of the optic nerve, stato-acoustic nerve, and the brain stem. Segmental demyelination is a cardinal lesion in leprosy, which is more common in peripheral nerves but it can involve any nerve of the body. The CNS is also not immune to leprosy because our observation suggests not only the involvement of the optic and stato-acoustic nerves but also of the brain stem. This hypothesis requires further strengthening by an extensive study of multimodality evoked potentials and MRI involving a larger number of patients. Histopathological and immunofluorescent studies of autopsy material of the brain may also contribute significantly for their confirmation.
REFERENCES
1. ANTIA, N. H, PANDYA, S. S. and DASTUR, D. K. Nerves in arm in leprosy. I. Clinical, electrodiagnostic and operative aspects. Int. J. Lepr. 38 (1970) 12-29.
2. AWASTHI, S., SINGH, G., DUITA, R. and PAHUJA, O. Audiovestibular involvement in leprosy. Indian J. Lepr. 62 (1990) 429-434.
3. BODDINGIUS, J. Ultrastructural changes in blood vessels of peripheral nerves in leprosy neuropathy. II. Borderline, borderline lepromatous and lepromatous patients. Acta Ncuropathol. 40 (1977) 21-39.
4. DASTUR, D. K., ANTIA, N. II. and DIVEKAR, S. C. The facial nerve in leprosy. 2. Pathology, pathogenesis, electromyography and clinical correlations. Int. J. Lepr. 34 (1966) 118-138.
5. DASTUR, D. K. and KABHOI.KAR, A. S. Histochemistry of leprous nerves and skin lesions. J. Pathol. 113 (1974) 69-77.
6. DECANDIA, A. and MARIANA, A. Studies of cochleo-vestibular function in patients with leprosy. Riv. Audiol. Prat. 1 (1965) 7-10.
7. DHARMENDRA. Diagnosis and general consideration. In: Notes on Leprosy. New Delhi: Ministry of Health, Government of India, 1954, p. 72.
8. EAMES, R. A. and LANGE, L. Clinical and pathological study of ischaemic neuropathy. J. Neurol. Neurosurg. Psychiatry 30 (1967) 215-226.
9. EGGERMONT, J. J. and SCHMIDT, P. H. The auditory brain stem response. In: Evoked Potential Manual. Colon, E. J. and Visser, S. L., eds. Dordrecht, The Netherlands: Kluwer Acadmic Publications, 1990, pp. 44-47.
10. EL.-ARINI, P. G., SHEHATA, N. A. and ABOU-ZEID, S. A. Eighth cranial nerve affection in leprosy. Int. J. Lepr. 38 (1970) 164-169.
11. ELLIOT, D. C. A report of leprosy lesions of the fundus. Int. J. Lepr. 16 (1948) 347-350.
12. GARG, V, MARKAND, O. N., el al. BAEP in IIMSN: site of origin of wave II. Neurology 32 (196)1017.
13. GUPTA, B. K. and KOCIIAK, D. K. Study of nerve conduction velocity, somatosensory-evoked potential and late responses (H-reflex and F-wave) of posterior tibial nerve in leprosy. Int. J. Lepr. 62 (1994)586-593.
14. HALLIDAY, A. M. Evoked potentials in clinical testing. In: Auditory Evoked Potentials in the Clinic. 2nd edn., McPherson, D. and Starr, A., eds. Edinburgh: Churchill Livingstone, 1993, pp. 359-382.
15. JEWETT, D. L. and WIUSTON, J. S. Auditory evoked farfields averaged from the scalp of humans. Brain 94 (1971) 681 -696.
16. KILNEY, P. and MAGATHAN, M. Predictive value of ABR in infants and children with moderate to profound hearing impairment. Ear Hearing 8 (1987)217-221.
17. KOWALSKI, J. W., RASHKVA, M. and ZAKREZEWSKA, B. Visual and brain stem auditory evoked potentials in hereditary motor-sensory neuropathy. Electromyogr. Clin. Neurophysiol. 31 (1991) 167-172.
18. KOYUNCU, M., CELIK, O., O/.TURK, A. and SAUNDERS, M. Audiovestibular system, fifth and seventh cranial nerve involvement in leprosy. Indian J. Lepr. 66(1994)421-428.
19. LATIF, S. The effects of certain skin diseases of the ear. nose and throat. M.Ch. degree thesis, Alexandria University, Egypt, 1967.
20. MILLER, D. H., NEWTON, M. R., VANDERPOEL, J. C. DUBOUI.AY, E. P. G. IL, HAI.I.IDAY, A. M., KENDALL, B. E., JOHNSON, G., MACMANUS, D. G., MOSELEY, I. F. and MCDONALD, W. I. Magnetic resonance imaging of the optic nerve in optic neuritis. Neurology 38 (1988) 175-179.
21. MOLLER, A. R. Evoked Potentials in Intraoperative Monitoring. Baltimore: Williams and Wilkins, 1988.
22. MUKHERJEE, R. and ANTIA, N. H. Organized nerve culture-a model for the study of nerve damage and cultivation of M. leprae. (Abstract) Int. J. Lepr. 52 Suppl. (1984) 734.
23. OZDAMAR, O., KRAUS, N. and CURRY, F. Auditory brain stem and middle latency responses in a patien with cortical deafness. Electroencephalogr. Clin. Neurophysiol. 53 (1982) 224-230.
24. PEDLEY, J. C, HARMAN, 1). J., WAUDBY, II. and MCDOUGALL, A. C. Leprosy in peripheral nerves: histopathological findings in 119 untreated patients in Nepal. J. Neurol. Neurosurg. Psychiatry 43(1980) 198-204.
25. PICTON, T. W., STAPELLS, I). R. and CAMPBELL, K. B. Auditory evoked potentials from the human cochlea and brain stem. J. Otolaryngol. 10 Suppl. (1981) 1-41.
26. PICTON, T. W., WOODS, S. L. and PROULX, G. B. Human auditory sustained potentials. I. The nature of the response. Electroencephalogr. Clin. Neurophysiol. 45 (1978) 186-197.
27. PRATT, H, SHENHEV, R. and GOLDSHER, M. Application of auditory evoked potentials to evaluate hearing disorders: assets and limitations. Israel J. Med. Sci. 21 (1985)44-49.
28. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966) 255-273.
29. POPPER, A. 11. and CHUTA, K. H. Evoked potentials in Guillain-Barre syndrome. Neurology 36 (1986)587-590.
30. SATYA MURTI, S., CACACE, A. T. and HANSON, P. A. Abnormal auditory evoked potentials in hereditary motor-sensory neuropathy. Ann. Neurol. 5 (1979)445-448.
31. SCHURING, A. G. and I.STRE, C. 0, JK. Hansen's disease and hearing. Arch. Otolaryngol. 89 (1969) 478-481.
32. SINGH, T, KAUR, S., KUMAR, B, SAWHNEY, B. B. and CHOPRA, J. S. A study of motor and sensory nerve conduction in leprosy. Indian J. Med. Res. 65 (1977)632-639.
33. SINGH, T. R., AGARWAL, S. K., BAJAJ, A. K., SINGH, R. K. and SINGH, M. M. Evaluation of audiovestibular status in leprosy. Indian J. Lepr. 56 (1984) 24-29.
34. SOMERSET, E. J. and SEN, N. R. Leprosy lesions of the fundus oculi. (Abstract) Int. J. Lepr. 24 (1956) 491-492.
35. SRINIVASAN, K. P. Hearing problems among leprosy sufferers. (Abstract) Int. J. Lepr. 52 Suppl. (1984)686.
36. THOMAS, P. K, WALKER, R. W. H, RUDGE, P., MORGAN HUGES, J. A., KING, R. II. M., JACOBS, J. M., MILES, K. R., ORMEROD, I. E. C, MURRAY, N. M. F. and MCDONALD, W. 1. Chronic demyelinating peripheral neuropathy associated with multifocal central nervous system demyelination. Brain 110(1987) 53-76.
37. UCHIDA, M. Atrophy of the optic nerve in leprosy. (Abstract) Int. J. Lepr. 9 (1941) 254.
38. UNCINI, A.. GALLUCI, M., LUGARESI, A., PORRINI, M. M., ONOFRI, M. and GAMBI, D. CNS involvement in chronic inflammatory demyelinating polyneuropathy: an electrophysiological and MRI study. Electromyogr. Clin. Neurophysiol. 31 (1991) 365-371.
39. VAN POOLE, G. M. Leprosy and tuberculosis of the eye. Trans. Am. Ophthalmol. Soc. 32 (1934) 596.
40. WHEELER, P. R. Metabolism in M. leprae: its relationship to other research on M. leprae and to aspects of metabolism in other mycobacteria and intracellular parasites. (Editorial) Int. J. Lepr. 52 (1984) 208-230.
41. WORTHINGTON, D. and PETERS, J. Quantifiable hearing and ABR: paradox or error? Ear Hearing 1 (1980)281-285.
1. M.D., M.A.M.S., D.N. (Vienna), Professor; Department of Medicine, S.P. Medical College, Bikaner 334 003, India.
2. M.D.; Department of Medicine, S.P. Medical College, Bikaner 334 003, India.
3. M.D.; Department of Medicine, S.P. Medical College, Bikaner 334 003, India.
4. M.D.; Department of Medicine, S.P. Medical College, Bikaner 334 003, India.
5. M.D., Department of Medicine, S.P. Medical College, Bikaner 334 003, India.
Reprint requests to Dr. D. K. Kochar, C54 Sadul Gunj, Bikaner 334 003, India.
Received tor publication on 20 February 1996.
Accepted for publication in revised form on 20 March 1997.