- Volume 65 , Number 2
- Page: 257–9
Regarding Brasil, et al .'s adverse effects in leprosy's WHO/MDT and paramedic's role in leprosy control program
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this JOURNAL is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the JOURNAL and thus interfere with its prime purpose.
To the Editor:
The June 1996 INTERNATIONAL JOURNAL OF LEPROSY (Vol. 64, pp. 97-104) published an article entitled "Results of a Surveillance System for Adverse Effects in Leprosy's WHO/MDT." In this article one of the hypotheses given by Brasil, et al. (1) to explain the low number of reported acute renal failure cases as an adverse effect of WHO/MDT was the fact that most of the leprosy program has been carried out by paramedics. We would like to make some comments regarding this article.
The state of Amazonas covers an area of 1,564,445 km2 in the north of Brazil. It is the largest state in the Amazon Basin with 2,424,817 inhabitants (1995) and its population density is a low 1.55 inhabitants/km2. Half of the population lives in the capital city, Manaus, and the rest are unevenly distributed between the interior towns (nearly 60%) and the rural areas.
The Instituto de Dermatologia Tropical e Venereología Alfredo da Matta is one of the National Reference Centres for Leprosy and the Reference Centre for Skin Diseases in the state. The management of the leprosy control program is the responsibility of this Institute.
The WHO/MDT in Amazonas started in 1982. The implementation was initiated by pilot studies in four areas: two areas in the capital at the Institute and in the Aleixo District, an ex-leprosarium which reported the first six cases of dapsone resistance in Brazil (6); two areas in the interior (in Lábrea and Tefé) both of which are municipalities with the highest prevalence and incidence rates for leprosy in the state. From the satisfactory results with the new therapeutic scheme, WHO/MDT was gradually implemented and expanded to all 62 municipalities of the state. In order to ensure efficient implementation of the new scheme, reorganization of the health infrastructure was necessary to enable the staff to cope with the revised strategy. When there were constraints, priorities were formulated so that available resources were put to the best use. Training of program personnel (physicians, nurses, social workers, biochemists, paramedics and laboratory technicians) has been carried out regularly in Manaus and in the interior over the past 10 years, with regular 5-to 10-day courses on general aspects of the disease, including its treatment. During the various courses, special attention is given to the rare but possible adverse effects of WHO/MDT. It is made clear to the participants that if they should come across any side effects they must interrupt the treatment, and this they have indeed done. They also are advised to refer the patients to the referral hospital in case of severe adverse effects or severe leprosy reaction, in addition to contacting the staff of the Institute by telephone in order to receive information about the correct procedures. More complicated cases that are unusual have, where necessary, been removed From the interior to Manaus by plane.
Most of the health centers and hospitals in the interior have two physicians who are responsible for the general medical assistance of the whole community. At these health units, the WHO/MDT has been administered, mainly by the paramedics.
Systematic and routine follow-up supervisionary activities of the health units in Manaus and in the interior have been carried out by the Institute's staff or From the regional health team.
From December 1982 to August 1996, a total of 20,019 patients were on or had completed WHO/MDT in Amazonas state. Of these, 10,864 (54.26%) received MDT in Manaus, where they could be monitored by physicians.
For the purpose of this letter, we are reporting 69 cases of adverse reactions (The Table) to WHO/MDT which resulted in treatment being interrupted by a physician in Manaus. These data can be compared with data collected in other regions where patients have been followed up by physicians (1-5).
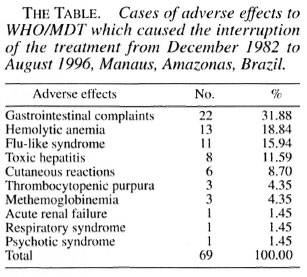
Regarding the progress of the patients who had their treatment suspended, 65 had their treatment re-introduced with alternative regimens, which excluded those drugs suspected of causing their adverse effects. One patient refused further treatment and 3 patients died (1 due to acute renal failure, 1 to thrombocytopenic purpura and 1 to hemolytic anemia). If, besides the case already reported, more cases of renal damage due to WHO/MDT had occurred in Manaus, they probably were mild cases and the patients probably had minimal symptoms which they did not complain about.
More than half of the leprosy cases in the state of Amazonas live in the capital and, therefore, have access to a physician. Taking this into consideration and also the results shown in The Table lead us to think that the hypothesis given in the article, regarding the role played by paramedics, cannot explain the reduced incidence of acute renal failure as a consequence of adverse reactions to MDT in the state.
From the large number of patients under WHO/MDT in Manaus, it could be said that the current regimen has remarkably few side effects. However, side effects should be diagnosed as soon as they arise and treated appropriately in order to prevent dire results.
In order to try to explain the greater number of acute renal failure cases observed in São Paulo compared with Manaus, new hypotheses need to be formulated and new studies should be carried out to verify them. It may be possible to include ethnic characteristics or differences in the levels of severity of the renal damage.
- Maria da Graça S. Cunha, M.D.
Head, Research and Training Division
- Antonio P. M. Schettini, M.D.
Emilia S. Pereira
Coordinator
Leprosy Control Program
State of Amazonas
- Valderiza L. Pedrosa
Epidemiologist
Head, Field Work Division
- Rossilene C. S. Cruz, M.D.
Megumi Sadahiro, M.P.H.
Instituto de Dermatologia Tropical e Venereología"Alfredo da Malta "
Rua Codajas n. 24
Cachoeirinha
Manaus, Amazonas, Brazil 69065-130
REFERENCES
1. BRASH., M. T. L. R. F., OPROMOLLA, D. V. A., MARZLIAK, M. L. C. and NOGUI;IRA, W. Results of a surveillance system for adverse affects in leprosy's WHO/MOT. Int. J. Lepr. 64 (19%) 97-104.
2. DEDHIA, N. M., ALMEIDA, A. F., KHANNA, V. B., MlTTAL, B. V. and ACHARYA, V. M. Acute renal failure-a complication of new multidrug regimen for treatment of leprosy. Int. J. Lepr. 54 (1986) 380-382.
3. GALLO, M. E. N., NERY, J. A. C. and GARCIA, C. C. Intercorrencias pelas drogas utili/adas nos esquemas poliquimioterapieos em hansem'ase. Hansen. Int. 20(1995)46-50.
4. GORDON, P. A., GRION, C. M. C., SOUSA, V., CARVALHO, V. P., DELFINO, V. D. A., MENDES, M. F., MATINI, A. M. and MocELINI, A. J. Insuficiência renal aguda pelo uso do esquema multidroga na hansem'ase. Hansen. Int. 17 (1992) 21-26.
5. RAMU, G. Toxic manifestations and side-effects of MDT regimens. Joint Meetings of Indian and Chemotherapy of Leprosy (THELEP) Scientists on Multidrug Therapy in Leprosy, Schieffelin Leprosy Research and Training Centre, Karigiri, India, 14-15 March 1988.
6. TALHARI, S., DAMASCO, M. H. S., CUNHA, M. G. S., SCHETTINI, A. P. and ADRADE, L. M. C. Sulfono-resistência secundária: comprovação laboratorial em seis casos. An. Bras. Dermatol. 60 (1985) 175-178.
7. Reprint requests to Dr. Cunha at the above address or FAX 55-92-663-31-55.