- Volume 65 , Number 2
- Page: 217–23
Monitoring steroid use in a field program; possible process indicators
ABSTRACT
Two new indicators are proposed in order to make the task of monitoring certain prevention of disability (POD) activities more straightforward. The indicators are very similar to the case detection rates and the cohort analyses already used in both leprosy and tuberculosis (TB) control; this makes them very simple to put into practice. Despite their simplicity, it is argued that these indicators can give important information about the implementation of POD activities in a routine field program and could, therefore, help in improving the quality of those services to patients. The indicators are the steroid start rate (SSR) and the steroid completion rate (SCR). A number of possible confounding factors have been looked at and they are not negligible. However, the case detection rates for new cases of leprosy and treatment completion rates for multidrug therapy (MDT) are subject to similar biases, which are well recognized and which have not detracted F rom the usefulness of these indicators in evaluating leprosy control activities. The POD indicators, if used with an awareness of the possible biases involved, can help to improve the quality of certain POD activities.RÉSUMÉ
Deux nouveaux indicateurs sont proposes afín de la rendre la tache de surveilance de certaines activités de prévention des incapacités (I'DT) plus directe. Les indicateurs sont très emblables aux taux de détection et aux analyses de cohortes utilisés aussi bien dans la lutte contre la lèpre que dans la lutte contre la tuberculose; ceci les rend très simple à utiliser. En dépit de leur simplicité, on argumente que ces indicateurs peuvent donner des informations importantes quant à la réalisation d'activités de prévention des incapacités dans un programme de contrôle de routine, et donc, aider à améliorer la qualité de ces services vis-à-vis des patients. Les indicateurs sont le taux de mise sous stéroïdes et le taux de traitement par stéroïdes terminé. Un certain nombre de possibles facteurs de confusion a été examiné; ils ne sont pas négligeables. Cependant, les taux de détection pour les nouveaux cas de lèpre et de traitement terminé pour la polychimiothérapie sont sujets à des biais semblables, qui sont bien reconnus et n'ont rien enlevé de l'utilité de ces indicateurs pour évaluer des activités de lutte contre la lèpre. Les indicateurs de PDI, s'ils sont utilisés en ayant à l'esprit les biais possibles, peuvent aider à améliorer la qualité de certaine activités de PDI.RESUMEN
Se proponen 2 nuevos indicadores tendientes a "monitorear" las activadades para la prevención de discapacidades, de manera directa. Los indicadores son muy similares a las tasas de detección de casos y a los análisis de cohortes ya usados en el control de la lepra y la tuberculosis; esto los hace muy simples de poner en práctica. No obstante su simplicidad, se argumenta que estos indicadores pueden dar información importante sobre las actividades de prevención de discapacidades en un programa de campo de rutina y podría, por lo tanto, ayudar a mejorar la calidad de esos servicios a los pacientes. Los indicadores son la tasa de inicio del tratamiento esteroidal y la tasa de terminación del tratamiento. Se ha puesto atención a un número de posibles factores de confusión y se ha visto que no son despreciables. Sin embargo, aunque las tasas de detección de casos (nevos) y las tasas de terminación de la poliquimioterapia están sujetas a distorciones similares bien conocidas, esto no limita la utilidad de los indicadores en la evaluación de las actividades de control de la lepra. El uso de los indicadores para la prevención de discapacidades, tomando en cuenta los posibles factores de distorción, puede ayudar a mejorar la calidad de las actividades orientadas a la prevención de discapacidades.The prevention of disability (POD) is recognized as the major challenge facing leprosy control programs, as the burden of cases requiring chemotherapy decreases sharply. There are three major phases of patient care, during each of which different efforts in POD are required.
The first phase, before the patient is started on treatment, requires good public education and awareness so that the new patients present for treatment early, before any detectable nerve damage has occurred.
In the second phase, that of chemotherapy with multiple drug therapy (MDT), the patients must be monitored to detect developing nerve function impairment as early as possible so that steroid treatment can be given; as an extension of this phase, patients must be made aware of the danger signs of new nerve damage so that they can attend for treatment, even if they have already finished their MDT.
In the third phase, patients with a residual, permanent impairment of nerve function must be helped to prevent further damage and disability through a variety of measures aimed at facilitating self-care. These may include the setting up of self-care groups for mutual encouragement, forms of socioeconomic rehabilitation that may allow the person to take a few days off From work to rest a blistered hand or foot, and some specific assistance such as the provision of appropriate footwear.
One of the major difficulties in the field of POD is that of measuring the results of the work done for the purposes of monitoring and evaluation. Evaluation is an essential part of the management cycle, and very little progress can be made in improving the quality of the services given if this part of the cycle is ignored. While individual patients or small cohorts of subjects in a research project are often very closely monitored, the outcome of POD activities for a program as a whole cannot at present be stated succinctly, nor with any degree of precision.
The first phase of POD can be usefully monitored by looking at the disability grades (DG) of new cases, the only POD indicator currently in use. Thus, the percentage of new cases presenting with grade 2 disability varies between 5% (or less) in programs with good public awareness and case-finding to around 20% or more in programs where there are serious delays in diagnosing new cases.
Evaluation of the second and third phases could be done directly by looking at the outcomes of a particular program, in this case, by quantifying the damage prevented in one patient or a group of patients by a particular intervention. Alternatively, the process of carrying out that intervention can be monitored, with the assumption that if it is carried out according to known guidelines a known outcome can be expected (3). This, in fact, is how MDT is monitored- the completion rate is only of value if it is assumed that fixed duration MDT gives a very high probability of cure. This may be a much simpler procedure to do in a routine control program and, therefore, a much more satisfactory method of monitoring POD activities.
This paper, therefore, suggests a possible mechanism for monitoring the process of disability prevention in phase two, namely, the treatment with steroids of acute neuritis in patients being seen regularly. For such a mechanism to be useful it must be easy and quick to carry out, it must lead to one or more indicators that can be compared between different programs (or in the same program over time), and it must measure something that is directly related to the prevention of disability.
Steroids are now well recognized as the treatment of choice for acute neuritis in leprosy which generally occurs as part of a type 1 or reversal reaction (RR) (8,9). Steroids also are widely used on an ambulatory basis for a variety of conditions in developed countries(13) The exact steroid regimen to be used for leprosy reactions-dosage, the procedure for step-wise reduction in dose, and duration-remains the subject of research and, in practice, may vary From program to program. A number of published studies, however, show that the majority of patients have a significant improvement in nerve function when treated with steroids (1,2,5-7,10,12).
The proposed method of monitoring relies on the quarterly reporting of patients started on steroids during that quarter and, at the same time, reporting on the completion of steroid treatment in a cohort of patients started on steroids 6-9 months previously. It is clear that this method is very similar to the way in which "new cases" and the "results of treatment" are reported for the chemotherapy of leprosy,-which makes it straightforward for staff already familiar with those report forms. The raw data are also very easily obtained From the treatment register where, in the ALERT program, any treatment with steroids is noted in red, alongside the MDT given.
A study of steroid treatment given during the year July 1994 to June 1995, within the ALERT leprosy control program area, was carried out in order to validate the proposed reporting mechanism and to calculate certain indicators From the results.
Background to ALERT leprosy control program. ALERT has managed leprosy control activities in the former Shoa Province of Ethiopia for over 20 years. There are over 250 leprosy clinics in an area of 85,000 sq. km. The population is just over 13 million (24% of the population of Ethiopia) and, apart From the years of serious civil unrest (1990-1991), the numbers of leprosy patients starting treatment each year have been very stable. For the year examined in this study the figure was 941 new cases. The prevalence (1566 patients on treatment at the end of the year) is 1.2 per 10,000 and the case detection rate is 7.1 per 100,000 ( ALERT Leprosy/TB Control Division 1995 Annual Report).
Treatment with steroids is governed by guidelines developed by Becx-Bleumink, et al. during the late 1980s (1,2). The steroid regimens all start with prednisolone 40 mg daily; this is decreased gradually to 0 in 14 weeks for PB cases and in 22 weeks for MB cases.
Every leprosy patient attending a clinic should have a voluntary muscle test/sensory test (VMT/ST) examination, the results being recorded on a printed form, so that changes can be monitored From month to month. Every 6 months, a review examination is entered into the patient's permanent record. If a patient is to be started on steroids, the prednisolone treatment report form (PTRF) is filled out, serving as a checklist for the required investigations (ALERT Manual for Field Treatment of Leprosy Reactions, 2nd edition, 1989). The examination findings at the end of steroid treatment should be entered on the reverse of the form.
Patients receiving prednisolone have the number of tablets given recorded in the ordinary patient register, usually in red, alongside their MDT record. Patients no longer receiving MDT are added to the register for the duration of their steroid treatment.
MATERIALS AND METHODS
All registers within the ALERT leprosy control program area were examined by one of us (NH-M) for the year July 1994 to June 1995, to extract information regarding the ambulatory treatment of patients with steroids. Patients referred to hospital for the start of steroid treatment (for example, children) were not identifiable From the registers.
In addition, patient records were examined to assess the results of steroid treatment in a conventional sense, namely, by looking for changes in the ST and VMT over time. The ST is carried out with a 10-gm monofilament, while the VMT is done using the scale strong : weak: paralyzed (S:W:P).
The result of treatment with steroids was taken From the examination findings at the end of the course of steroids or within 6 months of that date; if no examination details were found in the patient's record within that period, no result was recorded.
The effect of steroid treatment was considered "good" if more than 75% of the deficit in nerve function was recovered; "fair" means that recovery was between 50% and 75%; "poor" means that recovery was less than 50%, including no improvement. For example, suppose steroids were started for a recent complete loss of sensation (LOS) in both hands (10 points are tested in the hands, so 20 points of LOS were noted at the start). If sensation returns to 15 or more points, the result is "good"; if sensation returns to between 10 and 14 points, the result is "fair"; if sensation at the end is between 0 and 9 points, the result is "poor." With respect to VMT scores, a change From "P" to "W" is a 50% improvement, or "fair" result; a change From "P" or "W" to "S" is a 100% improvement, or "good" result.
The field staff were not informed in advance of this study so that the records were not "tidied up" in any way. The findings reflect the actual state of the records in a busy, routine program.
RESULTS
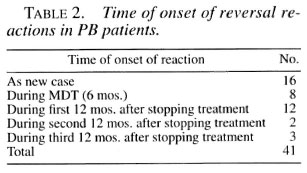
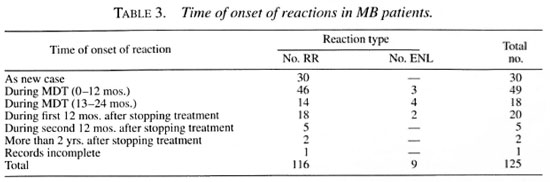
During the year studied, 166 patients (114 male and 52 female) were given steroids on an ambulatory basis. Table 1 shows the types of reactions treated according to the patient's classification. Table 2 shows the timing of these reactions in relation to the time of diagnosis and completion of MDT for paucibacillary (PB) patients. Table 3 gives information regarding the onset of both reversal reactions and erythema nodosum leprosum (ENL) reactions for multibacillary (MB) patients.
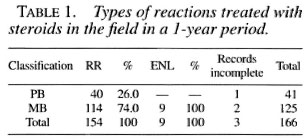
From the registers, it was simple to categorize patients according to their attendance for steroid treatment during the months after the onset of the reaction since the collection of steroid drugs is marked alongside the attendance for MDT. Table 4 shows the rates of treatment completion for 166 patients started on steroids for a reaction. If a patient fails to attend the clinic while on steroids every effort is made to trace him/her. Interruption of treatment for a short period is noted as treatment completed with one or more gap(s); in this sample, the gaps varied between 2 and 8 weeks. There was no difference between PB and MB patients in the rates of completion.
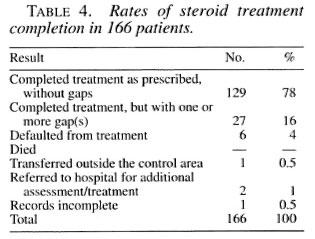
Although data collection proved very time-consuming, the outcome of steroid treatment in terms of nerve function was also assessed. Table 5 shows the results of this assessment. It will be noted that the number of incomplete records is unacceptably high, but this serves to illustrate the realistic position in a routine program.
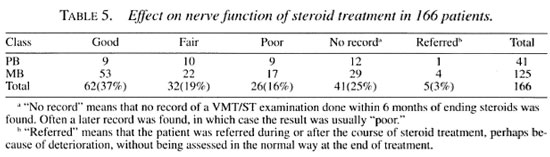
Of the 129 patients who completed treatment correctly, 56 (43%) had a "good" result; whereas only 6 (22%) of the 27 patients who had gaps in their treatment had a "good" result. This difference approaches statistical significance (Yates corrected ; χ2 = 3.35, p = 0.067) on this relatively small sample of patients.
DISCUSSION
The monitoring of POD activities in leprosy control programs is an important issue that has become more and more urgent as the prospect of "elimination of the disease as a public health problem" comes closer. If the work of disability prevention cannot be easily reported on and assessed, it is likely to decline in integrated and decentralized field programs; it certainly cannot be easily developed and improved, tasks that depend on monitoring and evaluation.
ALERT has managed a field program for many years and the possibility of field treatment of reactions was first studied here in the 1980s (1,2). It has proved difficult to monitor the effect on nerve function on a routine basis, and even when this is tried, much of the required data is not easily available (Table 5). While it may be possible in some places to enforce better recording of detailed nerve function assessments, it seems unlikely that this will be possible in an integrated setting. Even with the number of unavailable results, the majority (56%) of patients had a "good" or "fair" result From the current standardized steroid regimens.
Perhaps better results could have been expected in the ALERT program which has a number of years of experience with the field treatment of neuritis. A long-term field study carried out by ALERT is expected to shed light on this issue in the near future. From the preliminary figures, however, it seems likely that the results are much better in those patients who started treatment with DG 0 and who then developed neuritis. If patients already have nerve damage at the time of diagnosis, the staff cannot know for sure if this is recent or old and may give steroids in the hope of some improvement. It is possible that the ALERT staff have seen the value of steroids in many cases and are more willing to prescribe them for nerve damage of questionable duration. It has also been noted that some patients like to take steroids for their effect on mood and may, therefore, attempt to mimic a new deficit in nerve function. These reasons may explain why a number of patients fail to show any improvement with steroids.
Measuring the outcome of steroid treatment was time-consuming and incomplete. Reporting on the process may be much easier, namely, the number of cases started on treatment and the rate of completion of treatment. The results in terms of nerve function improvement can then be deduced From published studies while ongoing research may gradually improve the regimens used and refine the guidelines for treatment.
The following indicators can be easily calculated From the data presented here.
"Steroid Start Rate" (SSR). Reported quarterly, with annual totals, the SSR is calculated as follows:
(Cases started on steroids) / (Population at risk)
= 166/ 13,200,000
= 1.26 per 100,000 (for ALERT)
This indicator would be valuable for assessing POD activities From year to year in the same program. Comparisons with other programs, whether in the same country or elsewhere, would need to be made with caution, given the many factors which influence the number of patients developing and being treated for reactions. Epidemiological differences between programs which are likely to be important are: a) the true incidence of leprosy in the community; b) the case mix (ratio of PB:MB cases and number of single lesion cases); and c) the possibility of a backlog of old cases being started on treatment.
The risk factors for reactions and neuritis in individual patients are by no means fully elucidated, but they could also influence the SSR. They include the following, some being more important than others (4,11): a) the extent of clinical disease; b) the start of treatment with MDT; and c) pregnancy and lactation.
The following program-related factors may also play a part: a) the average time between the first symptoms of leprosy and the start of MDT; b) the average level of compliance with MDT; c) the threshold for referral of reaction cases to the hospital which may relate to a range of factors, such as availability of hospital beds, transport, distances and patient attitudes.
The criteria for diagnosing reactions and neuritis, and starting steroids, will vary From program to program and must be taken into account when comparing rates of starting steroids.
Interpretation of this indicator must be related to the knowledge of what is actually going on throughout the field program. Thus, if the SSR is, or becomes, very low it may suggest that too few patients are being treated with steroids, perhaps due to a lack of awareness, a lack of time to examine the patients properly in the clinics, or a lack of appropriate guidelines and charts. On the other hand, if the SSR is, or becomes, very high it may suggest that too many cases are being given steroids, perhaps due to changes (approved or unapproved) in the criteria for treatment. Any change in the rate would need investigation but would provide a warning that a routine practice at the clinic level may be changing.
If new policies come into force regarding case-finding or the diagnosis and treatment of neuritis, changes in the SSR are to be expected. However, given this background information for any particular program, the SSR (and any changes in it over time) will be a valuable additional indicator as to the implementation of POD activities in the field.
The case detection rate (CDR)-new cases started on treatment in a 1-year period divided by the population at risk-is obviously calculated in the same way as the SSR. The CDR is a useful indicator of the amount of leprosy in the community being studied, and is relatively stable over time. It would, therefore, be possible when comparing two programs within a country, for example, to look at the ratio of the two rates. As a straightforward indicator of how many reactions are being treated, however, the SSR should be quoted.
"Steroid Completion Rate" (SCR). reported quarterly, with annual totals, the SCR is a cohort analysis of those patients started on steroids 6-9 months previously and would be presented as a table, very much like Table 4. Supervisory and health education activities can then be targeted at improving the process of steroid treatment, with the assumption that if a high percentage complete their treatment as prescribed, the resulting nerve function improvement will be optimal.
It is clear that these two reports will not help in the management of the patients being reported on, as is also the case with the reports on new case detection and treatment completion in the chemotherapy of both leprosy and tuberculosis. What each of these reports aims to do is to monitor the activities of the program, so that problems can be identified and solved to the benefit of future patients. The treatment of patients at the clinic today can only be optimized by a knowledgeable health staff and good supervision carried out in the light of previous activity reports.
Acknowledgment. We thank Dr. Pieter Feenstra, Dr. Guido Groenen and Ms June Nash for their helpful comments, and Dr. Peter Byass for statistical advice.
REFERENCES
1. BECX-BLEUMINK, M. and BERHE, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the Leprosy Control Program of the All Africa Leprosy and Rehabilitation Training Centre (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
2. BECX-BLEUMINK, M., BERHE, D. and 'TMANNETJE, W. The management of nerve damage in the leprosy control services. Lepr. Rev. 61(1990)1-11.
3. DAVIES, H. T. O. and CROMBIE, I. B. Assessing the quality of care: measuring well supported processes may be more enlightening than monitoring outcomes. Br. Med. J. 311(1995)766.
4. DUNCAN, M. E. and PEARSON, J. M. H. Neuritis in pregnancy and lactation. Int. J. Lepr. 50(1982)31-38.
5. KIRAN, K. U., HOGEWEG, M. and SUNEETHA, S. Treatment of recent facial nerve damage with lagophthalmos, using a semi-standardized steroid regimen. Lepr. Rev. 62(1991)150-154.
6. KIRAN, K. U., STANLEY, J. N. A. and PEARSON, J. M. H. The outpatient treatment of nerve damage in patients with borderline leprosy using a semistandardized steroid regimen. Lepr. Rev. 56(1985)127-134.
7. LOCKWOOD, D. N. J., VINAYAKUMAR, S., STANLEY, J. N. A., MCADAM, K. P. W. L. and COLSTON, M. J. Clinical features and outcome of reversal (type 1) reactions in Hyderabad, India. Int. J. Lepr. 61(1993)8-15.
8. RICHARDUS, J. H. and SMITH, W. C. The risk of standardised regimens of corticosteroids for the treatment of leprosy reactions in the field. Lepr. Rev. 66(1995)328-329.
9. ROSE, P. and WATERS, M. F. R. Reversal reactions in leprosy and their management. Lepr. Rev. 62(1991)113-121.
10. TOUW-LANGENDIJK, E. M. J., BRANDSMA, J. W. and ANDERSEN, J. G. Treatment of ulnar and median nerve function loss in borderline leprosy. Lepr. Rev. 55(1984)41-46.
11. VAN BRAKEL, W. H. and KHAWAS, I. B. Nerve damage in leprosy: an epidemiological study of 396 patients in west Nepal. Part 1: Definitions, methods and frequencies. Lepr. Rev. 65(1994)204-221.
12. VAN BRAKEL, W. H. and KHAWAS, I. B. Nerve function impairment in leprosy: an epidemiological and clinical study. Part 2: Results of steroid treatment. Lepr. Rev. 67(1996)104-118.
13. WALSH, L. J., WONG, C. A., PRINGLE, M. and TAT-TERSFIELD, A. E. Use of oral corticosteroids in the community and the prevention of secondary osteoporosis: a cross-sectional study. Br. Med. J. 313(1996)344-346.
1. M.R.C.P., M.B.A.; Leprosy/Tuberculosis Control Division, ALERT, P. O. Box 165, Addis Ababa, Ethiopia.
2. Leprosy/Tuberculosis Control Division, ALERT, P. O. Box 165, Addis Ababa, Ethiopia.
Received for publication on 7 October 1996.
Accepted for publication in revised form on 29 January 1997.