- Volume 65 , Number 2
- Page: 224–9
Field trial on efficacy of supervised monthly dose of 600 mg rifampin, 400 mg ofloxacin and 100 mg minocycline for the treatment of leprosy; first results
ABSTRACT
In 1995, a field trial was implemented in Senegal in order to evaluate the efficacy of a regimen based on the monthly supervised intake of rifampin 600 mg, ofloxacin 400 mg and minocycline 100 mg to treat leprosy. During the first year of the trial, 220 patients with active leprosy (newly detected or relapsing after dapsone monotherapy) were recruited: 102 paucibacillary (PB) (60 males and 42 females) and 118 multibacillary (MB) (71 males and 47 females). All of them accepted the new treatment (none requested to be preferably put under standard WHO/MDT), no clinical sign which could be considered as a toxic effect of the drug was noted, and none of the patients refused to continue treatment because of any clinical trouble. The compliance was excellent: the 113 patients (PB and MB) detected during the first 6 months of the trial have taken six monthly doses in 6 months, as planned. The rate of clearance and the progressive decrease of cutaneous lesions was satisfactory. Although it is too soon to give comprehensive results, it should be noted that no treatment failure was observed in the 56 PB patients who have completed treatment and have been followed up for 6 months. The long-term efficacy of the new regimen is to be evaluated on the rate of relapse during the years following the cessation of treatment. If that relapse rate is acceptable (similar to that observed in patients after treatment with current standard WHO/ MDT), the new regimen could be a solution to treat, for instance, patients very irregular and/or living in remote or inaccessible areas since no selection of rifampin-resistant Mycobacterium leprae should be possible (a monthly dose of ofloxacin and minocycline being as effective as a close of dapsone and clofazimine taken daily for 1 month). Nevertheless, until longer terni results of this and other trials become available, there is no justification for any change in the treatment strateey, and ail leprosy patients should be put under standard WHOV MDT.RÉSUMÉ
En 1995. une étude expémentale a été mise en route au Sénégal pour évaluer l'efficacité d'un régime basé sur la prise mensuelle supervisée de 600 mg de rifampicine, 400 mg d'ofloxacine et 100 mg de minocycline pour le traitement de la lèpre. Pendant la première année de l'essai, 220 patients avec une lèpre active (nouveaux cas ou rechutes après monothérapie à la dapsone) ont été recrutés: 102 paucibacillaires (PB) (60 hommes et 42 femmes) et 118 multibacillaires (MB) (71 hommes et 47 femmes). Tous acceptèrent le nouveau traitement (aucun n'a demandé à être mis de préférence sous PCT/OMS), aucun signe clinique pouvant être considéré comme un effet toxique du traitement n'a été noté, et aucun patient n'a refusé de poursuivre le traitement à cause de problèmes cliniques. La régularité fut excellente: les 113 patients (PB et MB) détectés durant les six premiers mois de l'étude ont pris leurs six doses mensuelles en six mois, comme plannilié. Le taux de disparition et la diminution progressive des lésions cutanées furent satisfaisantes. Bien qu'il soit trop tôt pour donner des résultats complets, il faut noter qu'aucun échec thérapeutique n'a été observé chez les 56 patients PB qui ont terminé leur traitement et ont été suivis pendant 6 mois. L'efficacité à long terme du nouveau régime doit être évaluée sur base du taux de rechute durant les années qui suivent l'arrêt du traitement. Si ce taux de rechute est acceptable (semblable à celui observé chez les patients après traitement avec la PCT standard actuelle), le nouveau régime pourrait être une solution pour traiter, par exemple, des patients très irrèguliers ou vivant dans des régions éloignées ou inaccessibles, puisqu'aucune sélection de Mycobacterium leprae résistant à la rifàmpicine ne devrait être possible (une dose mensuelle d'ofloxacine et de minocycline étant aussi efficace qu'une dose quotidienne de clofazimine et de dapsone prise pendant un mois). Cependant, jusqu'à ce que des résultats à plus long terme ne soient disponibles pour cette étude et d'autres, aucun changement dans les stratégies thérapeutiques n'est justifié, et tous les malades de la lèpre devraient être mis sous PCT standard.RESUMEN
En 1995 se implemento un estudio de campo para evaluar la eficacia de un régimen de tratamiento basado en la administración supervisada de 600 mg de rifampina, 400 mg de olloxacina y 100 mg de minociclina, para el tratamiento de la lepra. Durante cl primer año del estudio se reclinaron 220 pacientes con lepra activa nueva o de recaída subsecuente al tratamiento con dapsona. Los pacientes fueron 102 casos paucibacilarcs, PB (60 hombres y 42 mujeres) y 118 casos multibacilares, MB (71 hombres, 47 mujeres). Todos los pacientes aceptaron el nuevo tratamiento, ningún paciente mostró efectos tóxicos derivados del uso de las drogas y ninguno de ellos se rehusó a continuar con el tratamiento. La constancia de los pacientes fue excelente: los 113 pacientes (PB y MB) detectados durante los primeros 6 meses del estudio habían, tomado 6 dosis mensuales en 6 meses, de acuerdo a lo planeado. La tasas de depuración y la disminución progresiva de las lesiones cutáneas fueron satisfactorias. Aunque es muy pronto para dar resultados concluyentes, debe hacerse notar que no se observaron fallas en los 56 pacientes PB que completaron el tratamiento y que fueron supervisados durante 6 meses. La eficacia de este tratamiento de larga duración está siendo evaluada para establecer la tasa de recaídas durante los años siguientes a la terminación del tratamiento. Si la tasa de recaídas es aceptable (similar a la que se observa en los pacientes tratados con la PQT estándar), el nuevo tratamiento podría ser una solución para tratar, por ejemplo, a pacientes muy irregulares, o a pacientes que viven en arcas remotas o inaccesibles, puesto que no se presentaría la emergencia de cepas resistentes a la rifampina (una dosis mensual de ofloxacina y minociclina es tan efectiva como una dosis de dapsona y clofazimina tomadas diariamente durante 1 mes). No obstante, hasta que no se tengan los resultados a largo plazo de éste y otros ensayos, no se justifica ningún cambio en la estrategia del tratamiento, y todos los pacientes con lepra deben someterse al tratamiento estándar con PQT.Since the beginning of the 1980s, as recommended by a World Health Organization (WHO) Study Group (13), multidrug therapy (MDT) for the treatment of leprosy has been widely implemented in all of the countries where the disease was still endemic. The drugs used in WHO/MDT are a combination of rifampin and clofazimine given monthly and clofazimine and dapsone given daily for multibacillary (MB) patients, and rifampin given monthly and dapsone given daily for paucibacillary (PB) patients. PB patients are considered as cured when they have taken 6 months of treatment and MB patients, 24 months (11). By 1994, some 5,660,000 patients were released From treatment with these combinations; the rate of relapse was less than 0.1 % per year in PB patients and less than 0.06% in MB patients (12).
In 1995, it was estimated that WHO/ MDT had prevented 500,000 to 1,000,000 cases of relapse and also 1 to 2 million patients From being physically disabled (11,14). Nevertheless, there is still a need to continue to search for new drug regimens in order to propose a simpler and more acceptable therapy, fully supervisable with flexible interval between doses, using the same drugs for both PB and MB patients. During the past few years, three drugs proved to have bactericidal activity against Mycobacterium leprae (2-6,15) and to be safe and well tolerated: ofloxacin, minocycline and clarithromycin. Because clarithromycin is known to cause gastro-intestinal side effects and is more expensive than the others, it was decided to keep it in reserve for treatment, in combination with ofloxacin and minocycline, of patients harboring rifampin-resistant M. leprae. The results of pilot trials conducted in previously untreated MB patients indicated that 22 doses of ofloxacin could be as effective as 2 years of clofazimine and dapsone (self-administered in the current WHO/MDT) in eliminating rifampin-resistant mutants (3). Therefore, a combination of rifampin, ofloxacin and minocycline given monthly under supervision would be effective and safe, cost-effective and operationally more simple.
In March 1995, a field trial was implemented in Senegal: all patients with active leprosy (newly detected or relapsing after treatment with dapsone alone) detected in five administrative regions were put on treatment with monthly doses of rifampin, ofloxacin and minocycline. The aim of this paper is to report on the results observed after 1 year.
MATERIALS AND METHODS
In Senegal, a country in West Africa of some 200,000 km2 with 8 million inhabitants in 1995, the leprosy control program is under the direction of a medical coordinator based at the direction of Public Health Services in Dakar, the capital city of Senegal. In each administrative region a medical doctor (physician) is responsible for all public health activities, including leprosy activities. There are 10 administrative/medical regions in the whole country. In each region (divided into three medical districts), two to three specialized nurses and a laboratory technician are in charge of the medical activities of the program.
The Institut de Léprologie Appliquée (ILAD), which is part of the Public Health Services, is also located in Dakar. The Institute is responsible for the development of a field applied research program in leprosy and for the training of physicians, specialized nurses and other technicians involved in the leprosy program. It is also in charge of the specialized care of leprosy patients hospitalized because of reaction and/or neuritis or any other complications of the disease. Seven physicians, including an epidemiologist who is head of the Research Unit, work full time at the Institute.
For the purpose of the present study, the specialized nurses involved in the control program in the Dakar region and the four regions close to Dakar were trained at the Institute. The objectives of the study were explained to them and standardization of the work was decided. Once a month, the principal investigator (PI) of the study, or his assistant, was to visit the region he was in charge of in order to examine the subjects suspected to be leprosy patients and presented by the specialized nurse. The diagnosis of leprosy was based on a clinical examination of the skin and large nerve trunks and a search for acid-fast bacilli (AFB) in earlobes and skin lesions and determination of the bacterial index (BI) (8). In addition, a skin biopsy for pathological examination was taken and also a blood sample for the detection of antibodies to HIV. When the diagnosis of leprosy was confirmed, the patient was given each month, under supervision of the PI or his assistant, a dose of rifampin 600 mg, ofloxacin 400 mg and minocycline 100 mg (half-dose for children below 30 kg body weight). Patients were excluded From the trial (and given standard WHO/MDT) when they were under 5 years or over 65 years of age, pregnant, or had pulmonary tuberculosis, cancer, diabetes, hepatic or cardiac disease, or epilepsy.
Treatment compliance was assessed on the regularity of intake of the prescribed doses: all patients having taken 24 doses within 36 months if MB or 6 doses within 9 months if PB were considered as regular patients.
The efficacy of the treatment was evaluated on the rate of treatment failure (while on treatment) and on the occurrence of relapse during the 5-year period following the cessation of treatment. They are defined as: for an MB patient, the appearance of new lesion(s) and an increase of the BI of at least 2+ at any site; for PB, the appearance of new skin and/or nerve lesion(s), and/or a BI of at least 2+ at any site.
RESULTS
During the last 2 months of 1994 and the first 2 months of 1995, the PI and his assistant visited the five regions of the trial and, in each of them, met the physician responsible for public health activities. Everyone responsible was given the objectives of the study and received a copy of the protocol. In addition, all specialized nurses and health workers who had been trained at the Institute were visited in each medical district. As a result, the recruitment of patients for the trial began during the second half of March 1995.
Between March 1995 and March 1996, 252 subjects suspected to be leprosy patients were examined; 240 of them were confirmed to have active leprosy. Twenty of them were excluded From the trial (age over 65 years or below 5 years) and 220 (131 males, 89 females) were put on treatment (Table 1). Among the 220 patients, 102 were PB (46%), 94 of whom were new patients (never treated) and 8 were patients relapsing after dapsone monotherapy, and 118 were MB patients (99 newly detected patients and 19 relapsing after dapsone monotherapy). Of these 118 patients, 62 (13 BL and 49 LL) had a BI of > 4+. The distribution of the patients according to age, sex, and type of leprosy (PB, MB) is given in Table 1. There were 34 children below the age of 15, that is 15.5% of the whole group of 220 patients.

Of the 220 patients, 125 (57%) had disability at the time of detection, 73 of whom had grade 1 according to WHO classification (11) and 52 had grade 2. All of these 52 patients had isolated or combined chronic plantar ulcers, claw hands or lagophthalmos. Leprosy reactions were observed in 40 patients (11 PB, 29 MB) on detection, and in 12 others (2 PB and 10 MB) during the first 6 months of treatment. Of the total 52 patients with reaction, 30 suffered severe reaction and had to be hospitalized at ILAD for treatment, 19 on detection and 11 during the first 6 months of treatment (Table 2).
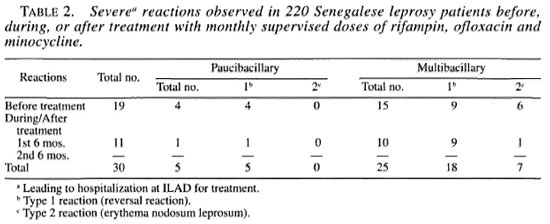
All of the patients meeting the inclusion criteria accepted the new treatment. No clinical sign which could be considered as a toxic effect of the drug was noted, either at first intake of the drug combination or at any of the subsequent doses. None of the patients refused to continue treatment because of clinical symptoms due, or claimed to be due, to the intake of the drug combination. The compliance to treatment was excellent, and out of the 102 PB patients put on treatment, the first 56 who have taken the six doses in 6 months were put under surveillance. Also, the first 57 MB patients put on treatment during the first 6 months of the trial have taken six doses in 6 months. In all of the PB and MB patients the intake of the rifampin + ofloxacin + minocycline (ROM) combination resulted in evident and rapid improvement of the lesions. In 25% of the 56 PB patients who have completed treatment, the lesion had disappeared after the intake of six doses, and in the last 75%, the lesion(s) had dramatically decreased (in size and volume as well as in number when they were multiple at detection). Also, deflation of the lesions and progressive repigmentation were observed in MB patients who had completed the first year of treatment.
DISCUSSION
The results of the present trial demonstrate that a fully supervised monthly regimen combining rifampin, ofloxacin and minocycline was easily implemented in Senegal. First of all, the treatment based on the monthly intake of drugs was very well accepted by the patients; none of them requested to be put under standard WHO/ MDT rather than the new regimen. Secondly, no side effect was observed in any of the 220 patients put on treatment; none of them requested to stop treatment because of symptoms imputable (or supposed to be) to the drug intake. Also, the compliance to treatment was excellent since the 113 PB and MB patients detected during the first 6 months of the trial have taken six monthly doses in 6 months, as planned. Finally, the majority of reactions were observed at the time of detection before treatment. During the first 6-month period of treatment, severe reactions (leading to hospitalization at ILAD for treatment) were observed in 8.5% of MB patients and in 2% of PB patients, percentages similar to those reported in patients under WHO/MDT (1). All these results, which may be considered as excellent, allow us to predict that the inclusion and follow up of the patients recruited during the second year of the trial will be carried out without difficulty.
As defined in the protocol, the efficacy of the ROM monthly doses treatment is to be evaluated in terms of treatment failure and/or relapses, and it is certainly too soon to give comprehensive results. Nevertheless, to date, no treatment failure was observed in the 56 PB patients who have completed treatment and have been followed up for 6 months. Also, the rate of clearance and the progressive decrease of cutaneous lesions in PB patients was satisfactory and, again, quite comparable to that described in the earliest reports From leprosy patients put under WHO/MDT(9-10). Regarding MB patients, the number of them having completed the first 12 months of treatment is quite small, and it is not presently possible to evaluate the decrease in the average BI after 12 monthly doses of the ROM combination.
One last point, apparently surprising, needs to be clarified: the high proportion of MB patients among the study population. Of the 220 patients with active leprosy included into our trial, 118 (54%) were MB when it is usually estimated that, in West Africa, that proportion does not exceed 30%-40%. It must be kept in mind that such estimation was made some decades ago when BT patients with a BI of 1+ were considered as PB. In our study, 17 patients were classified as BT patients at the pathological examination but were considered as multibacillary (BB) since the search for AFB was positive. In fact, if using the criteria for classification valid until the early 1980s, the proportion of MB patients would have been 45% in our study population.
One of the most important benefits to be expected From the new intermittent regimen is that the elimination of rifampin-resistant mutants of M. leprae does not rely upon a daily nonsupervised intake of drugs as is the case in the current WHO/MDT. Since 22 doses of ofloxacin are as effective as 2 years of dapsone and clofazimine taken daily in the current WHO/MDT (3), no selection of rifampin-resistant mutants should be possible in the patients taking the proposed new regimen, even in those who are very irregular. Therefore, if the rate of relapse after completion of treatment is acceptable (similar to that observed in patients after treatment with current standard WHO/MDT), the new regimen could be a solution to treat (for instance) patients living in remote or inaccessible areas. This could be even more the case if, as suggested by results of trials conducted in nude mice (7), the duration of treatments (containing rifampin which remains the most powerful bactericidal drug against M. leprae plus either daily dapsone and clofazimine or monthly doses of new drugs) could be significantly shortened.
Acknowledgment. The drugs used in the study were provided by Laboratoires Roussel Uclaf, 93235 Romainville, France, and Laboratoires Wyeth Lederlé, Puteaux 92031 Paris La Défense, France.
REFERENCES
1. BIRCH, M. C. Leprosy treatment in Nepal with multidrug regimens. Lepr. Rev. 55(1984)255-264.
2. GELBER, R. HSiu, P. and TSANG, N. Clarithromycin at very low level and on intermittent administration inhibits the growth of M. leprae in mice. Int. J. Lepr. 60(1992)485-487.
3. GROSSET, J.-H. Traitement antibactérien de la lèpre. In: La Lèpre. Sansarricq, IL, Coord. Paris: Ellipses, 1995.
4. GROSSET, J.-H, GUELPA-LAURAS, C. C , PERANI, E.G. and BEOLETTO, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
5. JI, B., JAMET, PPERANI, E., BOBIN, P. and GROSSIT, J.-H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J. Infect. Dis. 168(1993)188-190.
6. JI, BPERANI, E. G. and GROSSET, J.-H. Bacterici dal activities of single or multiple doses of various combinations of new antileprosy drugs and/or ri fampin against M. leprae in mice. Int. J. Lepr. 60(1992)485-487.
7. JI, BPERANI, E. GPETINON, C. and GROSSET, J.-H. Bactericidal activities of combination of new drugs against Mycobacterium leprae in nude mice. Antimicrob. Agents Chemother. 40(1996)393-399.
8. RIDLEY, D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Baltimore: Williams and Wilkins, 1964, pp. 620-622.
9. ROSE, P. Short-course multidrug therapy for paucibacillary patients in Guyana: preliminary com munication. Lepr. Rev. 55(1984)134-147.
10. SAMUEL, N. M., SAMUEL, S., NAKAMI, N. and MURNU, R. Multidrug treatment of leprosy-practical application in Nepal. Lepr. Rev. 55(1984)265-272.
11. WHO ACTION PROGRAMME FOR THE ELIMINATION OF LEPROSY. A guide for the elimination of leprosy as a public health problem. Geneva: World Health Organization, 1995.
12. WHO LEPROSY UNIT. Risk of relapse in leprosy. Geneva: World Health Organization 1994. WHO/CTD/LEP.94.1.
13. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
14. WHO STUDY GROUP. Chemotherapy of leprosy. Geneva: World Health Organization, 1994. Tech. Rep. Ser. 746.
15. XIONG, J-H., JI, B., PERONI, E. and GROSSETT, J.-H. Further study on the effectiveness of single doses of clarythromicin and minocycline against Mycobacterium leprae in mice. Int. J. Lepr. 62(1994)37-42.
1. Mane, M.D.; Institut de Leprologie Appliquée, B. P. 11023, Dakar CD Annexe, Senegal.
2. J.-L. Cartel, M.DInstitut de Leprologie Appliquée, B. P. 11023, Dakar CD Annexe, Senegal.
3. J.-H. Grosset, M.DProfessor, Département de Bactériologie-Virologie, Faculté de Médecine Pitie-Salpetriere, 91 Blvd. de l'Hopital, 75634 Paris 13, France.
Received for publication on 27 May 1996. Accepted for publication in revised form on 28 December 1996.