- Volume 65 , Number 3
- Page: 305–19
Trends in leprosy case detection rates
ABSTRACT
Background: A systematic review of the trends in leprosy incidence is lacking. The question of whether leprosy transmission has declined remains, therefore, unanswered. This study investigates trends in new case detection rates (NCDRs) in selected leprosy-endemic areas f rom different continents. Methods: A literature search using specific inclusion criteria was performed. Average annual rates of change in NCDRs were obtained by exponential curve fitting. The variation in trends within individual areas was investigated using direct and indirect information on leprosy control activities. Results: This review covers 16 areas in the Pacific, Asia, Africa and Latin America. For 10 out of the 16 areas, the trend was seen to be declining consistently over the last 10 years or longer. Near stabilization or stabilization after decline was observed for two areas. For three areas, interpretation of recent NCDRs was difficult due to changes in control, but two of them showed a decline over the study period. A consistently increasing trend was observed over the last 20 years in the one remaining area. The observed downward trends could not be attributed to reduced control activities or changed diagnostic criteria. A general acceleration of downward trends in the NCDR after the introduction of multidrug therapy (MDT) has not so far occurred. Conclusion: Our main conclusion is that despite many differences between the studies and study areas, the review demonstrates a considerable tendency of downward NCDR trends. Lack of information and changing control conditions necessitate caution in interpreting NCDR trends in individual areas. A general impact of MDT on NCDR trends is so far not visible. The coming years will be crucial for MDTbased control to prove its ability to reduce leprosy incidence.RÉSUMÉ
Contexte: On manque d'une revue systématique des tendances de l'incidence de la lèpre. La question de savoir si la transmission de la lèpre a diminué reste donc non résolue. Cette étude analyse les tendances de taux de détection de nouveaux cas dans des régions sélectionées, endémiques pour la lèpre, de différents continents.Méthodes: Une recherche de littérature a été réalisée an utilisant des critères spécifiques d'inclusion. Les taux annuels moyans de modification des taux de détection ont été obtenus en les ajustant à des courbes exponentielles. Les variations dans les tendances à l'intérieur des régions ont été analysées en utilisant des informations directes et indirectes relatives aux activités de lutte contre la lèpre. Résultats: Cette revue couvre 16 régions dans le Pacifique, l'Asie, l'Afrique et l'Amérique latine. Pour 10 de ces 16 régions, on a observé que la tendance était à la diminution au cours des 10 dernières années ou sur une plus longue période. Une situation proche d'un état stable ou une stabilisation après diminution a été observée pour deux régions. Pour trois régions, l'interprétation des taux de détection récents était difficile en raison de modifications dans les méthodes de hutte, mais deux d'entre elles ont montré une diminution au cours de la période d'étude. Une tendance à l'augmentation a été observée au cours des 20 dernières années dans la dernière région. Les tendances à la diminution observées ne pouvaient pas être attribuées à une diminution des activités de lutte ou à un changement des critères diagnostiques. Une accélération générale de la diminution des taux de détection après l'introduction de la polychimiothérapie (PCT) n'est jusqu'à présent pas survenue. Conclusion: Notre conclusion principale est que, en dépit de différences nombreuses entre les études et les régions d'étude, la revue démontre une tendance considérable à la diminution des taux de détection. Le manque d'information et les changements dans les conditions de lutte contre la lèpre rendent la prudence nécessaire dans l'interprétation des tendances dans chaque région. Les prochaines années serent cruciales pour les activités de lutte basées sur la PCX, pour prouver leur capacité de réduire l'incidence de la lèpre.RESUMEN
Antecedentes: Debido a que no existen estudios sistemáticos sobre las tendencias en la incidencia de la lepra, la pregunta relativa a la declinación de la transmisión de la enfermedad permanece sin respuesta. En este estudio se investigan las tendencias de las tasas de detección de casos nuevos de lepra (TDCN) en áreas seleccionadas de diferentes continentes donde la enfermedad es endémica. Métodos: Se realizó una investigción de la literatura usando criterios específicos de inclusión. Las tasas promedio anual izadas de los cambios en las TDCN se obtuvieron utilizando curvas de ajuste exponencial. La variación en las tendencias dentro de áreas individuales se investigó usando información directa e indirecta sobre las actividades de control de la lepra. Resultados: El estudio abarcó 16 áreas en el Pacífico, Asia, Africa y América Latina. En 10 de las 16 áreas se observó una declinación constante en la incidencia de la enfermedad durante los últimos 10 años.En dos áreas se observó una estabilización después de la declinación. En 3 áreas, la interpretación de las TDCN fue difícil debido cambios en los programas de control, aunque en dos de ellas se notó una declinación en el periodo de estudio. En el área restante se observó una tendencia al aumento en los últimos 20 años. Las tendencias a la disminución observadas, no pudieron atribuirse a reducciones en las actividades de control ni a cambios en los criterios de diagnóstico. La introducción de la poliquimoterapia (PQT) todavía no ha conducido a la aceleración general de las tendencies a la disminución en las TDCN. Conclusión: Nuestra conclusión principal es que a pesar de las diferencias entre los estudios y las áreas de estudio, la revisión demuestra una considerable tendencia a la declinación en las TDCN. Sin embargo, la falta de información y las cambiantes condiciones de control requieren de gran cautela en la interpretación de las TDCN en áreas individuales. Todavía no es apreciable un impacto general de la PQT sobre las TDCN. Los próximos años serán cruciales para que el control basado en la PQT pruebe su capacidad para reducir la incidencia de la lepra.The prevalence of leprosy, as measured by number of cases registered for treatment, and the new case detection rate (NCDR) are the conventional indicators for monitoring trends in leprosy control and elimination programs.
Dapsone was the only available drug for several decades. In dapsone-based programs, treatment duration was variable, often for 10 years or even lifelong. Registered prevalence would be cumulative figures affected by mortality and migration of patients.
From the early 1980s onward, dapsone has gradually been replaced by the more effective multidrug therapy (MDT). Under MDT, the leprosy elimination goal has been formulated as a reduction in prevalence to a level below 1 per 10,000 population by the year 2000 (27). There will only be a direct relationship between registered prevalence and NCDRs when new cases are put on treatment and when the recommended MDT treatment duration does not change over time. Under this condition, trends in registered prevalence and in NCDRs will differ little during the MDT era.
Trends in NCDRs reflect trends in incidence rates provided that no significant changes occur in case detection efforts, self reporting behavior, or diagnostic procedures and criteria. Incidence is here defined as the first appearance of clinically detectable signs which would lead to the diagnosis of "leprosy." The NCDR does not depend upon the duration of treatment. Therefore, the NCDR is a better indicator than registered prevalence for monitoring trends in transmission over extended time periods during which both dapsone- and MDTbased programs have been carried out.
Crude worldwide data suggest that the number of newly detected cases has remained roughly constant for 10 years. It stood around 560,000 in 1994 (42). The present paper provides an overview of trends in NCDRs for different areas of the world, which were selected on the basis of peer-reviewed publications satisfying quality and completeness criteria. Other information, e.g., on changes in case detection efforts and on the impairment status of newly detected cases, is used in order to assess whether trends in NCDRs indeed rellect trends in incidence. The impact of the main interventions-early detection, chemotherapy and BCG vaccination, which generally has been rationalized as a preventive measure against tuberculosis but which may be equally or more effective against leprosy (13)-on NCDR trends is discussed. This discussion includes the possible consequences of MDT introduction on incidence.
MATERIALS AND METHODS
The publications for this review were selected as follows. A literature search in Medline from 1986 onward was performed. A publication was selected as a candidate for the review when its abstract in Medline made reference to information on new case detection, incidence or the prevalence of leprosy. Candidate publications for which the denominator of the NCDRs was well defined and for which newly detected cases were representative for the new case load in an area were included in the next selection step. Examples of rejections are publications on new cases which were by majority imported, or on new cases reporting to a hospital in an area where also other case detection activities took place. Only publications which presented a series of at least 10 NCDRs over a period including 1986 and covering at least 10 years of continuous case detection, or which contained references pertaining to the same study which present these series, were maintained in the last selection step. The only source used apart from scientific journals is the proceedings of the meeting on "Leprosy profiles with special attention to MDT implementation" (Tokyo: Sasakawa Memorial Health Foundation, 1991).
In order to interpret the observed NCDRs and to assess whether their trends reflect trends in incidence, the selected journal publications and proceedings were scrutinized for information on (changes in) other epidemiological indicators and (changes in) operational aspects of control programs. Relevant information includes population size and density; the profile of newly detected cases (impairment and disability status, single lesion proportion, type index, smear positivity); mean age at detection and age specific new case detection rates (both by year of detection and by year of birth); prevalence rates at the start and end of the study period; occurrence of relapses; diagnostic criteria and procedures; delay until detection; drug regimens used; organization of control and the continuity of control services over time (e.g., case detection methods, transition from a vertical to a horizontal control program, history of BCG vaccination) and, finally, evidence for socioeconomic development.
Spearman's rank correlation coefficients between calendar year and NCDR were calculated. Exponential curves were fitted to the NCDRs over the period covered by the time series. The explained proportion of the variation in the time series, the R2 coefficient for the fitted curves, was calculated. R2 is a measure of goodness of fit of the curve to the data.
RESULTS
The journal publications and proceedings which were selected cover 16 areas, namely, 7 Asian, 5 African and 3 Latin American areas and French Polynesia (Pacific Islands). The number of cases detected, population size and NCDR study period are given in Table 1. The prevalence rate and NCDR at the start and at the end of the study period vary widely among the areas under review (Table 2). The study period exceeds 25 years for six areas.
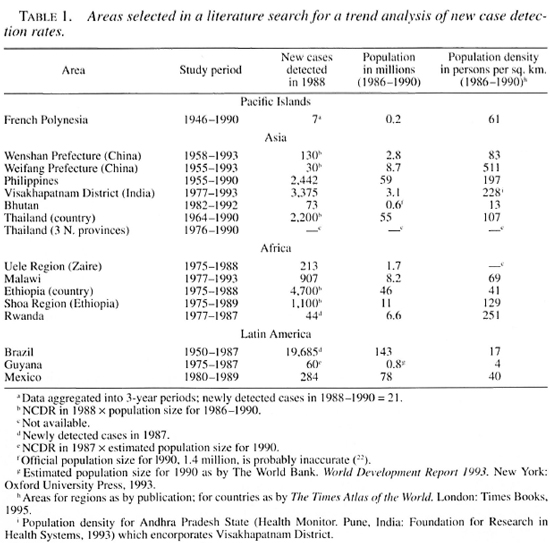
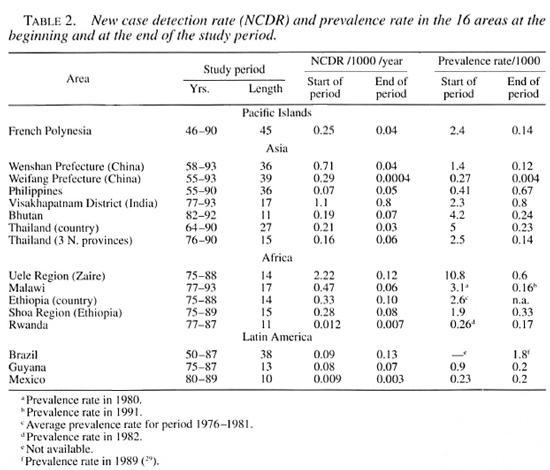
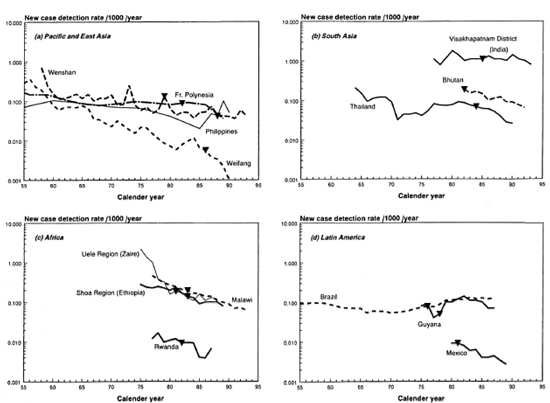
The Figure. Trend in the new case detection rate (NCDR) for 14 of the 16 areas covered by this review. Not given are the three northeastern provinces of Thailand and Ethiopia, which show trends similar to Thailand and the Shoa Region in Ethiopia. Rates from before 1955 were only available for French Polynesia and Brazil. The rates for the period 1991-1993 for Weifang are smaller than 0.001 per 1000 per year.  = introduction of treatment regimens containing rifampin, usually MDT.
= introduction of treatment regimens containing rifampin, usually MDT.
The NCDRs for all areas are plotted on a logarithmic scale (The Figure). On visual inspection, a downward trend can be observed for most areas. In areas with monotonous declines, trends are (about) linear on a logarithmic scale. This supports the use of exponential curve fitting of the data (i.e., constant proportional annual changes in the NCDR). Curve fitting confirmed the decline of the NCDR for all areas except Brazil and Guyana, which show small increases in the NCDR (Tables 3 and 4).
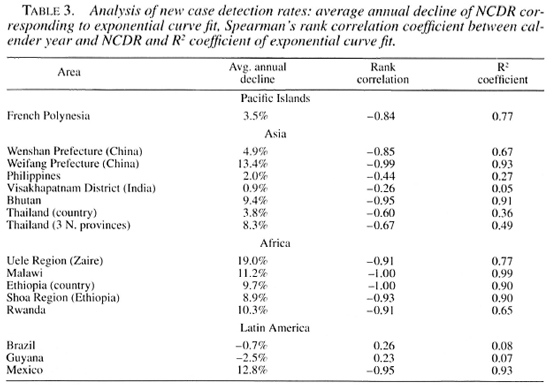

The five areas with the smallest rates of decline, below 5%, are situated in Asia and the Pacific: French Polynesia, Wenshan Prefecture (China), the country of Thailand, The Philippines and in Visakhapatnam District, India. The declines for all other areas exceed 8%, with a maximal decline of 19% for the Uele Region in Zaire (Table 3).
In concordance with the curve fitting results, all Spearman's rank correlation coefficients are negative, except for Brazil and Guyana (Table 3). For the small average annual changes of the NCDR, natural fluctuations are expected to be an important source of variation in the data. It is, therefore, not surprising that Brazil, Guyana, and the areas with lower declines also have smaller Spearman's rank correlation coefficients and R: coefficients. The relatively high coefficients of French Polynesia can be explained from the aggregation of data into 3year periods, which dampens much of the year-to-year variability.
The individual areas are analyzed here in more detail and related to available information on control programs and case detection methods.
Pacific Islands
French Polynesia (7, 8). A new leprosy control program was implemented in French Polynesia by the end of the 1940s. The NCDR declined between 1946 and 1967 and remained roughly stable until 1987. Transmission induced by relapses of nearly half of the multibacillary (MB) patients after dapsone monotherapy might have contributed to the stabilization according to Cartel, et al. (7). The leprosy control program organizes active case finding among household contacts and passive case finding. MDT was introduced in 1982, resulting in intensified active case finding in household contacts and improved management and follow up of patients. The relapse rate with MDT so far was nil. The NCDR clearly decreased in the last 3-year period, 1988-1990.
Asia
Wenshan Prefecture (China) (25). Leprosy control was initiated in the late 1950s as part of the national program. In the first years, much effort was placed on case finding, possibly explaining the higher NCDRs of 1958 and 1959. A downward trend was observed over the period 1962-1993. The two upward peaks of the NCDR coincide with the establishment of a network of eight county skin disease control stations (1973) and the introduction of rifampin in combination with dapsone (1979). The beginning of MDT in 1986 was followed by increased control activities, and no clear decline in the NCDR is visible since.
Weifang Prefecture (China) (24, 25) A leprosy control program was initiated in 1955. A consistem decline in NCDRs is observed with some elevations coincidingwith so-called clue surveys for ShandongProvince to which Weifang betones. Thesesurveys were carried out in 1955-1958, 1965-1966, 1971-1972, 1975-1976 and 1983-1984. More intensified case finding took place in the 1980s. MDT was introduced in 1986. Only 10, 9, 5, 4 and again 5 new cases were detected in the successive years between 1989 and 1993 in a population of 8.7 million in 1992. These recent cases were mainly self reporting.
The Philippines (1) Case finding methods were not explicitly described. Five-year NCDRs were available for 1955-1985 and also annual data for 1987 to 1990. Leprosy control activities were resumed from 1946 onward. Mobile skin clinics were established between 1955 and 1959. Only a small decline of the NCDR was observed over the years 1955-1975, followed by a more rapid decline over the decade 1975-1985. Transition from a vertical control system to an integrated health service approach took almost two decades, but was completed before the start of MDT in 1988. The NCDR has risen sharply during 1986-1989, i.e., just before and during MDT introduction.
Visakhapatnam District (India) (17) and Report on the workshop on impact of MDT on trend of leprosy. Madras: Indian Association of Leprologists, 1994). Data were presented from mid-year to mid-year; The Figure displays the year 1976-1977 as the calender year 1977. A significant downward trend is not observed. The early peak in the NCDR in 1980 remains unexplained. Both the proportion of voluntary reporting cases among total new cases and the NCDR for actively detected cases among the examined population (annual decline 4.5%) showed important fluctuations. General population surveys are the most important component of active case finding. It is also carried out through contact surveys and school surveys. MDT was introduced in 1984-1985.
Bhutan (22). A vertical program had already been operational for some years before the establishment of the National Leprosy Control Programme in 1981. MDT was introduced in 1982 and data were presented from 1982 onward. The population of Bhutan had a natural growth rate during the study period, but there was also largescale emigration. The NCDRs were, therefore, based on the same population size for all years. Contact and group surveys continued throughout. Mass surveys were held on a regular basis until 1988 and were subsequently gradually replaced by focal surveys in areas of previously known high prevalence. The proportion self reporting, the proportion with disability, and the proportion with high smear positivity among newly detected cases increased during the study period (22). Interpretation of the quite regular downward trend in the NCDR is, therefore, in our opinion not straightforward. The MDT coverage was below 10% in 1982 and above 80% from 1988 onward, with a coverage of 89% by the end of 1992.
Thailand (30, 31). A specialized program was established in Thailand in 1955, and revision to an integrated program started in 1970. A sharp decline in the NCDR was observed in 1971. The integration was completed in 1977, covering 67 of the 73 provinces of Thailand. The NCDR increased from 1972 onward, peaked in 1981, and declined ever since, with an average annual decline of 10.0% for the period 1979-1990 as compared to 3.8% for the study period as a whole. During the years 1984-1990, MDT gradually replaced dapsone monotherapy. Household contact surveillance, rapid village surveys (mobile clinics), school surveys, and skin clinic services have been practiced regularly throughout, although the degree of intensity of some of these methods varied over time. Three highly endemic northeastern provinces for which the specialized program approach was maintained have similar trends as Thailand as a whole (31).
Africa
Uele Region (Zaire) (37, 38). The leprosy control system was interrupted from 1964 to 1974 and was gradually reorganized afterward. Case finding is passive; the control program includes up to 20 mobile teams that contact patients monthly. The NCDR fell sharply in the initial years after 1974, which might be due to a reduction of false-positives in case ascertainment ("). An initial backlog in case detection cannot be excluded. For the period 1980-1988, exponential curve fitting gives an average annual decline of 5% as compared to 19% for the study period as a whole. Combined treatment regimens, containing rifampin, have been administered from 1981 onward. Leprosy control activities have been progressively integrated into a structure of primary health care introduced in 1983.
Malawi (5, 6, 32)Methods of leprosy control work during 1973-1983 have been described by Boerrigter and Ponnighaus (5). A mobile service organized patient treatment and passive case finding through periodic leprosy clinics. School surveys covered most pupils at least once during 1974-1983. A review of patients on treatment along with the introduction of MDT in 1983-1984 caused a great reduction in the prevalence rate. As a consequence, the number of staff-and self reporting opportunities according to Boerrigter and Ponnighaus (6)-were reduced. Otherwise, detection activities have remained comparable throughout 1973-1993 (6, 32). The decline of the NCDR is strikingly regular over the entire study period.
Shoa Region (Ethiopia) (2). Case detection was almost exclusively passive. Leprosy clinics were either run in general health services or, if nonexistent, in other settings. The observed average decline for the study period as a whole is largely caused by a drop in the NCDR over the period 1980-1985. MDT was introduced in 1983. The pattern for Ethiopia as a whole (3) is similar to that of the Shoa Region, but the paper presents less information on control.
Rwanda (36). A nongovernmental organization organized leprosy control in cooperation with the authorities from 1964 to 1984. MDT was introduced in 1982. Exponential curve fitting up to 1984 gives an average annual decline of 5.3% as compared to 10.3% for the study period as a whole. The overall average annual decline can, hence, for an important part be attributed to the dip in the years 1985-1986. With respect to this dip, Stes and Malatre (36) stated: "The steep dip during 1985-6, the years of transition, may be due to diminished control activities while the Service National de Lutte Contra la Lèpre was being set up. The 1987 rise could then be seen as a 'catching-up' manoeuvre of previously undetected cases, and is probably a good sign." This implies that the overall average annual decline should be interpreted with caution. The National Service particularly insists on the integration of leprosy control with primary health care. Case detection in Rwanda is based on voluntary reporting at dispensaries or health services on visits of mobile units. Information campaigns are organized and new patients are invited to bring their children and household contacts for examination.
Latin America
Brazil (26, 29). Significant upward or downward trends in NCDRs for the period 1950-1987 were not observed. During 1950-1968, when data registration was poor, policy guidelines were not clear, and personnel training and motivation were deficient, the NCDR declined by 3% per year (:"). Integration of leprosy control activities into primary health care was started by the end of the 1960s. From 1969 to 1987, control programs were improved by the implementation of technical guidelines, decentralization of case finding and case holding and better logistic facilities. The NCDR increased by 6% per year during the period 1969-1987 (R2 = 0.92, Spearman's rank correlation = 0.96). Region-wise analysis revealed that the observed increase affected the entire country(26). A combination of dapsone and rifampin was given to all MB cases from 1976 onward; gradual implementation of MDT for all new cases started in 1986.
Guyana (34). Case funding methods are not described explicitly. The present Guyana Leprosy Control Programme began in 1971. Multibacillary patients received dapsone, clofazimine and rifampin together from 1978 onward. The NCDR increased in the late 1970s following program expansion on receipt of external budgetary support. It peaked directly after the introduction of MDT in December 1981 and declined afterward. The low NCDRs of the early years and the subsequent increase cause the average annual increase of the NCDR.
Mexico (10). Case finding methods have not been described explicitly. The vertical National Leprosy Control Program was started in I960, and was incorporated into the state health services in 1981. At that time, treatment started to include rifampin and clofazimine in several different combinations. The organization of operations was, in this phase, somewhat irregular. In 1989, the program had a national office with several functions, including supervision. A comprehensive program for leprosy control is carried out in the zone with the highest prevalence. The Mexican NCDR showed a quite regular fall during the study period (1980-1989). A plan for the implementation of MDT was developed in 1989 by the National Leprosy Control Office.
DISCUSSION
Overall pattern
The data from all three continents and the Pacific Islands show a downward trend in the NCDR for most areas considered. The magnitude of the decline does not seem to depend on the population size, population density, or leprosy endemicity level-the latter apparently also being independent of population size and population density-of the area (Tables 1, 2 and 3).
Marked differences can be observed in the rate of decline between areas and also within areas over the study period. For most areas, including all African areas, this rate exceeds 8%. The rate of decline is below 5% in 4 out of the 5 areas in Asia and the Pacific Islands with study periods longer than 25 years (Tables 2 and 3). Clearly, it was difficult to achieve a sustained decline over long time periods in these areas. Publication bias also cannot be excluded in the sense that time series over longer periods are more prone to be published, whereas short-term trends might only be published in case of success, i.e., clearly declining trends.
Passive and active case detection
The NCDR decline did not depend upon case detection methods, but it was more variable in areas where case detection involved active components (e.g., household contact surveys, school surveys and general population surveys). Active case finding was often used in Asia and the Pacific Islands. Passive case finding was generally most important in the African areas.
Departures from overall trend
In some areas, like the Uele Region (Zaire) and Wenshan Prefecture (China), sharp initial declines in the NCDR were observed. This might be an artifact caused by detection of many "old" prevalent cases during the first years of leprosy control programs. Only later, newly detected cases will be mostly cases having contracted leprosy recently. If the first years are disregarded, most areas show declines that continue over the full study period OR at least over the last decade.
A less favorable picture emerges in three areas where the NCDR remained constant during long periods (French Polynesia, Wenshan Prefecture, Visakhapatnam District) and in two areas with no evidence of a consistent trend during the study period (The Philippines and Guyana). Relapses in French Polynesia were already mentioned. In Wenshan, control activities were increased after the implementation of MDT. A steady pattern over the study period is observed in Visakhapatnam. In Guyana, the NCDR increased following program expansion ON receipt of external budget support, and peaked directly after the introduction of MDT. Intensified case finding must have caused the exceptional rise in the NCDR just before and during MDT introduction in The Philippines. Clear peaks in new case detection at MDT introduction also have been reported from, e.g., Madagascar (42). For Visakhapatnam, the absence of a clear peak in the NCDR at MDT introduction can be explained by a phased introduction of MDT.
A distinctly different picture is observed in Brazil: its NCDR increased throughout the entire country during 1969-1987. Considering the clear increase in trends in Brazil and its individual regions and because a lower rate of NCDR increase was observed for lepromatous plus borderline cases than for tuberculoid cases, Motta and Zuniga suggest that the increase in the NCDR is not only the consequence of improved awareness by health units, but also reflects a real increase in incidence (26). The Brazilian Coordinator for Sanitary Dermatology comments that the main operational changes that influence the data did happen after 1986, and supports the suggestion that epidemiological factors contribute to the increase in the NCDR in Brazil up to 1987 (Maria Leide de Oliveira, personal communication). We are not aware of consistently increasing trends in the NCDR in other areas.
Operational factors and declining trends
Operational factors might have been responsible for declines in the NCDR. Reductions in case detection efforts are of particular concern. Reductions have not been reported explicitly, although detection methods changed in Bhutan (replacement of mass surveys by focal surveys). Integration of leprosy control activities into the general health services might also lead to a reduction IN case detection efforts. The NCDR was already falling at the time of integration in the Uele Region in Zaire and in Rwanda. The transition period toward integration (1970-1977) coincides with reduced NCDRs in Thailand: the reduction was, however, temporary. Reductions IN health personnel in the leprosy program did not coincide with the sharp declines in the NCDR in Malawi (6).
An increase in impairments and disabilities in an existing control program deserves attention since it might suggest late diagnosis and, hence, reduced control activities. Information on this aspect was incomplete. A definite (but slight) increase in the impairment and disability status of new patients in areas with persistingly declining trends was seen only in Bhutan. The proportion with a high smear positivity among newly detected cases in Bhutan also increased (22). Insufficient information prohibited an analysis of the proportion of single lesion cases among newly detected cases, which is an indicator for the share of active case detection efforts.
Indicator for declining incidence: increasing age at detection
In Thailand, the mean age at detection increased by nearly 7 years over the last 15 years of the study period while new case detection decreased. Age shifts under declining endemicity were observed in Nigeria, Japan, Venezuela (20), Portugal (21), Norway (19) and Shandong Province in China (24). The age shift occurred under varying levels of NCDR and could already be discerned after 10 years of declining incidence IN, e.g., Nigeria and Japan. The age shifts in Thailand, Norway and Shandong Province were of the same order.
The study of trends in age-specific detection rates is more informative. In Thailand, detection rates declined in all age groups between 1976-1980 and 1986-1990. The highest detection rates were observed for age groups older than 35 throughout 1976-1990. During this period, the maximum case detection level first occurred in the age group 45-54 and, in later years, in the age group 55-64. Declining rates for onset of disease for all age groups together with an increasing relative risk for the oldest as compared to the youngest age group were, over time, observed in Portugal (21), Norway (19) and Shandong Province (24). Interestingly, increases in age at onset were not observed over time for these three areas when rates were analyzed by year of birth. Details by year of birth were not available for Thailand.
A shift to detection at older ages combined with increased disability might point to longer delays in detection. However, in Thailand the proportion of patients with impairment and disability did not show a clear increase. This suggests that the age shift in detection is not the consequence of longer delays in detection alone and, thus, reflects a real age shift in incidence. For the other areas with persistently declining trends, information on the age at detection was either not available, or of limited usefulness because of only a short time-period or small numbers involved, or not representative because of increasing proportions of children being examined.
Type index among new cases
The type index (proportion of lepromatous or MB among newly detected cases) has been advocated as an indicator for changing trends in leprosy incidence [Report on the group discussions on the needs and prospects for epidemiological tools in leprosy control. WHO workshop on epidemioloszy of leprosy in relation to control. Lepr. Rev. 63 (1992) 114s-122s.]. This index is, however, sensitive for classification criteria and case detection efforts. Reduced case detection efforts can lead to an increase in the type index because of both self cure and downgrading of tuberculoid (or paucibacillary) cases. An increasing type index during intensification of control has also been observed (Wenshan Prefecture after MDT implementation). In addition, long-term declines of leprosy have been observed together with both an increasing and a decreasing type index (20, 21). In the present review, the behavior of the type index among newly detected cases also showed much variation, which could not easily be explained.
Variation between studies
The reviewed studies differ in many aspects. Examples are: terminology used, level of detail of available information, length of study periods, leprosy endemicity levels and applied case detection methods, and control strategies over time. NCDRs from the studies were, therefore, analyzed separately with respect to control conditions and underlying trends. One should be careful in directly comparing the trend statistics (Table 3) of the areas.
Do trends in the NCDR reflect trends in underlying incidence?
According to the 1988 WHO Expert Committee definition, a case of leprosy is defined as a person showing clinical signs of leprosy, with or without bacteriological confirmation of the diagnosis, and requiring chemotherapy (40). Clinical diagnosis is commonly based on the three cardinal signs of leprosy: anesthetic skin lesion(s), enlarged peripheral nerve(s) and the presence of Mycobacterium leprae in slit-skin smears or in nasal mucus scrapings (18). Diagnosis is not always straightforward. The establishment of loss of sensation in skin lesions can, for example, be difficult. Changes in diagnostic criteria and procedures, in the quality of these procedures, and in case detection efforts (including alertness for leprosy) affect NCDRs. Larger proportions of new cases with very early leprosy having single lesions, with questionable diagnostic specificity and a tendency toward self-healing, can be expected in the case of active case detection when surveys are undertaken at shorter intervals (16). Changes in certain administrative and managerial decisions, such as targets for case detection and incentives for MDT activities, are also expected to influence NCDRs (17). The above considerations indicate that trends in NCDRs not necessarily reflect trends in underlying incidence. Although evidence was hardly available in the reviewed papers, changes that might prohibit extrapolation of trends in NCDRs to trends in incidence cannot be excluded. Information on the occurrence of relapses and its possible inclusion of relapses in NCDRs is also sparse in the reviewed papers.
Selection of papers
A possible publication bias in the sense of a tendency to publish on high-quality control programs covering long periods of time or indicating successful leprosy control was already mentioned, and cannot be excluded in a review of this type.
The inclusion criteria aimed at selecting papers which report on data that have been collected regularly over time in specific areas. Less refined data are available for large geographical regions and although they should be interpreted cautiously, they are definitely important (42). Between 1993 and 1994, new case detection rates for WHO Regions as a whole decreased by 4.5% and 7.8% for, respectively, the Americas and South-East Asia, and increased by 20.8%, 25.9% and 3.3% for, respectively, Africa, the Eastern Mediterranean and the Western Pacific. A reduced NCDR in India explains much of the South-East Asian decrease. Considerable improvement in case detection as a consequence of setting the leprosy elimination target is reported for the African and Eastern Mediterranean Regions. Worldwide leprosy detection has remained about constant over the period 1985-1995 (42).
Interaction with tuberculosis
Exposure to other mycobacteria and the risk for leprosy are probably associated. It has, in particular, been argued that M. tuberculosis protects against M. leprae (12). In the Leprosy Prevention Trial (Tamil Nadu, India), however, tuberculin positivity had only very limited influence on the susceptibility for leprosy (M. D. Gupte, unpublished data from LPT). A study of a possible correlation between trends in leprosy and tuberculosis calls for a thorough analysis of the appropriate additional information on tuberculosis incidence-tuberculin surveys might be more informative than tuberculosis NCDRs-and on the continuity of data collection activities and tuberculosis control activities.
Role of socioeconomic development
It is generally believed that leprosy incidence declines with improving socioeconomic standards (14). The NCDR was already below 1:100,000 in 1985 in Weifang and a further decline in Weifang was steep in the period 1985-1993, while there was no clear decline in Wenshan. During the same period, the economic development is said to have been somewhat slow in Wenshan, whereas Weifang experienced rapid growth (29). The gross prefectural product per capita showed a highly significant negative correlation with both the prevalence rate and the NCDR over the period 1985-1991 in both Weifang and Wenshan Prefectures. The correlation of these rates with average annual income over this period is also negative for Weifang, but positive for Wenshan. The latter finding is ". . . likely due to the increased control activities in Wenshan since the implementation of MDT in 1986 . . ." (25) Examples of declines in the incidence rate coinciding with rapid socioeconomic development from the literature are: Japan (excluding Okinawa), Okinawa itself (although later) and Taiwan (35). Okinawa Prefecture had the slowest rate of economic development in Japan(41).
Role of bcg vaccination
BCG vaccination has been shown to provide protection against leprosy. Protective efficacies from 20% to 80% have been reported (13). Hence, BCG is expected to prevent new leprosy infections and, subsequently, new sources of infection. BCG vaccination was mentioned for 8 out of the 16 areas in this analysis. Details on vaccination programs are, however, not given and assessment of their impact is therefore not possible. Referring to detection being generally low in children and high in adults, with low detection in indeterminate leprosy, BCG is argued to have played some role in leprosy morbidity reduction in Weifang Prefecture (25). This might, however, be questioned because the proportion of ages 0-14 among new cases had been declining in the successive 5-year periods between 1955-1959 and 1975-1979 in Shandong Province (incorporating Weifang Prefecture) (24); whereas Shandong initiated BCG vaccination only in the 1970s.
Role of chemotherapy
Chemotherapy is thought to have played a role of its own in several areas. Becx-Bleumink identifies dapsone monotherapy as the most probable reason for the decline in Ethiopia (2). The motivation is that BCG coverage of newborns was less than 25% in the 1980s and probably not higher in the 1970s and earlier; whereas, in addition, improvement in the socioeconomic conditions of the rural Ethiopian population during the last decades would at most have been very marginal. A second example is the possible role of chemotherapy control in the initial decline of the NCDR (1946-1966) in French Polynesia where economic development really only started after 1962, and where systematic BCG vaccination was introduced by the mid-1960s (7).
The impact of chemotherapy is generally difficult to assess because socioeconomic development, BCG vaccination, and control through chemotherapy often go hand in hand. MDT is at present, however, regarded as the mainstay for leprosy control by WHO (27). The rationale for this WHO policy is clear. Early diagnosis and effective treatment do not only cure individual patients, but may significantly reduce leprosy transmission. This requires a drug regimen that is easily accepted by leprosy patients, such as MDT, which became available in the 1980s. The advantages of MDT include the absence of treatment failures due to drug resistance, very low relapse rates following completion of treatment, fixed and relatively short duration of treatment, and very low frequency of side effects (27). MDT improves patient compliance, encourages early self reporting, motivates health workers, and can induce a considerable upgrading of leprosy control activities (11).
Dapsone monotherapy renders patients noninfectious within a reasonable short period of time but lacks the favorable indirect effects of MDT. An acceleration of declining trends in NCDRs after MDT introduction might, therefore, largely be attributed to these indirect effects of MDT. The combined use of its main bactericidal drug, rifampin, with other drugs preceded the introduction of MDT in some areas. Accelerations of declining trends after the introduction of these combined regimens or MDT can be observed only in the country of Thailand and in Weifang Prefecture, where case finding intensified in the 1980s and where the NCDR was already very low at MDT introduction in 1986 (The Figure). In French Polynesia, where the NCDR had stabilized, the NCDR dropped in the last 3-year period, 5 years after MDT introduction.
Skepticism on the possible impact of chemotherapy exists, and the literature provides some examples. A stabilization of NCDRs which could not be attributed to operational factors was observed in the dapsone-bascd programs of two Leprosy Control Units of the Gandhi Memorial Leprosy Foundation (Sevagram, T. Narsipur) (In retrospect & prospect. Wardha: Gandhi Memorial Leprosy Foundation, 1974). A controlled attempt to assess the impact of drug therapy (rifampin) on incidence failed to show any effect after 5 years in Myanmar (12). Another finding relates to tuberculosis: similar declines in tuberculosis prevalence were reported from a special intervention (with chemotherapy) area and the "control" area where no special treatment facilities had been introduced (15).
Various explanations can be given for the absence of accelerations under MDT. The incubation period of leprosy is not well known but usually believed to be several years, implying that it might still be too early to see pronounced accelerations. Reductions in transmission also might be masked by increased case detection efforts as part of MDT implementation policies (e.g., The Philippines). It is also possible that not yet detected cases are responsible for transmission. This might imply that detection is too late to reduce transmission much, possibly even to the extent of prohibiting an impact of leprosy chemotherapy on transmission. A related observation is an average period between the patient's first observation of signs of leprosy and diagnosis of 2.3 years in Ethiopia (July 1987-July 1989) (2). The delay until detection in Wenshan Prefecture was between 2 and 5 years for 24% and longer than 5 years for 8.2% of newly detected cases (period 1986-1993) (25).
WHO is also studying the relationship between incidence and new case detection (42). Preliminary results of collaborative studies indicate that the majority of cases are detected late, even in programs that have used MDT for many years: ". . . in the majority of countries, only a small proportion of newly detected cases (10%) are true incident cases; about 75% of newly detected cases started 3-5 years earlier and about 15% arc detected 5 to 10 years after the onset of the disease." The gap between the estimated number of cases and those actually registered for treatment is said to be very large in some countries (Bangladesh, Indonesia, Viet Nam, Mali, Niger and Sudan). Big gaps between estimated and registered prevalence were also noticed in places in India, where sample surveys were undertaken or where intensive case detection campaigns were carried out even after 4 years of MDT implementation. A study in six subcenters, however, revealed a large proportion of cases detected during surveys to be cases of "early leprosy" (17).
Several other mechanisms for transmission have been mentioned. These include subclinically infected persons (23), carriers of M. leprae in the nose within endemic populations (9, 23, 28, 29), and animal reservoirs and the presence of M. leprae in the soil (4). It has been hypothesized that everyone in an endemic population will harbor M. leprae at some time, and that clinical leprosy arises from a pool of subclinical infection and not by transmission from an individual index case (33). If some of these factors indeed play a role in transmission, then early detection and chemotherapy treatment of cases might very well be insufficient to have a major impact on leprosy trends. The importance of these factors is, however, not clear and, at present, cannot be established for want of appropriate investigation tools.
Acknowledgment. The present study was taken up in the context of a collaborative project for the development of a simulation model for leprosy at Erasmus University Rotterdam and CJIL FIELD Unit. Financial support for this project by the Netherlands Leprosy Relief Association (NSL) and the WHO Action Programme for the Elimination of Leprosy is gratefully acknowledged.
REFERENCES
1. ABELLA, J. E. AND CARRILO, M. P. Leprosy control in The Philippines. In: Leprosy Profiles with Special Attention to MDT Implementation. Tokyo: Sasakawa Memorial Health Foundation, 1991, pp. 41-53.
2. BECX-BLEUMINK, M. Priorities for the future and prospects for leprosy control. (Editorial) Int. J. Lepr. 61 (1993) 82-101.
3. BERHE, D., HAIMANOT, R. T., TEDLA, T. and TADDESSE, T. Epidemiological pattern of leprosy in Ethiopia: a review of the control programmes. Lepr. Rev. 61 (1990) 258-266.
4. BLAKE, L. A.. WEST, B. C, LAKY. C. H. and TODD, J. T. Environmental nonhuman sources oHeprosy Rev. Infect. Dis. 9 (1987) 562-577.
5. BOERRIGTER, G. and PONNIGHAUS, J. M. Ten years' leprosy control work in Malawi (Central Africa). I. Methods and outcome after treatment. Lepr. Rev. 57 (1986) 199-219.
6. BOERRIGTER, G. and PONNIGHAUS, J. M. Does the introduction of WHO-MDT influence trends in the incidence of leprosy? The Malawian experience. Lepr. Rev. 64 ( 1993) 227-235.
7. CARTEL, J. L., BOUTIN, J. P., SPIEGEL, A., GLAZIOU, P., PLICHART, R., CARDINES, R. and GKOSSET, J. H. Leprosy in French Polynesia; epidemiological trends between 1946 and 1987. Lepr. Rev. 63 (1992) 211-222.
8. CARTEL, J. L., SPIEGEL, A., NGUYEN NGOC, L., MOULIA-PELAT, J. P., MARTIN, P. M. and GROSSET, J. H. Leprosy in French Polynesia; the possible impact of multidrug therapy on epidemiological trends. Lepr. Rev. 63 (1992) 223-230.
9. DE WIT. M. Y., DOUGLAS. J. T., MCFADDEN, J. and KLATSER, P. R. Polymerase chain reaction for detection of Mycobacterium leprae in nasal swab specimens. J. Clin. Microbiol. 31 (1993) 502-506.
10. DOMINGUEZ, J. R. and GARCIA, F. C. Leprosy control program in Mexico. In: Leprosy Profiles with Special Attention to MDT Implementation. Tokyo: Sasakawa Memorial Health Foundation. 1991, pp. 87-92.
11. FEENSTRA, P. Needs and prospects for epidemiological tools in leprosy control. Lepr. Rev. 63 Suppl. (1992) 3s-l0s.
12. FINE, P. E. Leprosy: the epidemiology of a slow bacterium. Epidemiol. Rev. 4 (1982) 161-188.
13. FINE, P. E. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 44 (1988) 691-703.
14. FINE, P. E. Reflections on the elimination of leprosy. (Editorial) Int. J. Lepr. 60 (1992) 71-80.
15. FRIMODT-MOLLER, J.. ACHARYULU, G. S. and KESAVA Pn LAI, K. A controlled study of the effect of a domiciliary tuberculosis chemotherapy programme in a rural community in south India. Indian J. Med. Res. 73 (1981) 1-80.
16. GUPTE, M. D. Early diagnosis of leprosy under field conditions. Indian J. Lepr. 65 (1993) 3-12.
17. GUPTE, M. D. Elimination of leprosy: forecasts and projections. Indian J. Lepr. 66 (1994) 19-35.
18. HASTINGS, R. C, ed. Leprosy. Edinburgh: Churchill Livingstone. 1985.
19. IRGENS, L. M. Leprosy IN Norway; AN epidemiological study based on a national patient registry. Lepr- Rev. 51 Suppl. (1980) ls-l.30s.
20. IRGENS, L. M. Secular trends in leprosy: increase in age at onset associated with declining rates and long incubation periods. Int. J. Lepr. 53 (1985) 610-617.
21. IRGENS, L. M., MELO CAEIRO, F. and LECHAT, M. F. Leprosy IN Portugal 1946-80: epidemiologic patterns observed during declining incidence rates. Lepr. Rev. 61 (1990)32-49.
22. JAKEMAN, P., JAKEMAN, N. R. and SLNGAY, J. Trends in leprosy in the Kingdom of Bhutan, 1982-1992. Lepr. Rev. 66(1995) 69-75.
23. KLATSER, P. R., VAN BEERS, S., MADJID, B.. DAY. R. and DE WIT. M. Y. Detection of Mycobacterium leprae nasal carriers in populations for which leprosy is endemic. J. Clin. Microbiol. 31 (1993) 2947-2951.
24. LI, H. Y, PAN, Y. L. and WANG, Y. Leprosy control in Shandong Province, China. 1955-1983; some epidemiological features. Int. J. Lepr. 53 (1985)79-85.
25. LI. H. Y. WENG, X. M., Li, T., ZHENG. D. Y. MAO, Z. M., RAN, S. P. and Liu, F. W. Long-term effect of leprosy control in two prefectures of China, 1955-1993. Int. J. Lepr. 63 (1995) 213-221.
26. MOTTA, C. P. and ZUNIGA, M. Time trends of Hansen's disease in Brazil. Int. J. Lepr. 58 (1990) 453-461.
27. NOORDEEN, S. K. Elimination of leprosy as a public health problem: progress and prospects. Bull. WHO 73 (1995) 1-6.
28. PATTYN, S. R., UKSI, D., IEVEN, M., GRILLONE, S. and RAES, V. Detection of Mycobacterium leprae by the polymerase chain reaction in nasal swabs of leprosy patients and their contacts. I tit J. Lepr. 61 (1993) 389-393.
29. PENNA. G. O. and PLKEIKA. G. F. M. Leprosy control in Brazil. In: Leprosy Profiles with Special Attention to MDT Implementation. TOKYO: Saskawa Memorial Health Foundation, 1991, pp. 64-83.
30. PLRAYAVARAPORN, C. and PEERAPAKORN, S. Leprosy CONTROL in Thailand. In: Leprosy PROFILES with Special Attention to MDT Implementation. Tokyo: Sasakawa Memorial Health Foundation, 1991, pp. 54-59.
31. PLRAYAVARAPORN, C. and PEERAPAKORN, S. The measurement of the epidemiological impact of multidrug therapy. Lepr. Rev. 63 Suppl. (1992) 84s-92s.
32. PONNIGHAUS, J. M. Leprosy. The beginning of an end to a public health problem? Dermatol. Clin. 13(1995)525-536.
33. REICH, C. V. Leprosy: cause, transmission, and a new theory of pathogenesis. Rev. Infect. Dis. 9 (1987)590-594.
34. ROSE, P. Changes in epidemiological indices following the introduction of WHO MDT into the Guyana leprosy control programme. Lepr. Rev. 60 (1989)151-156.
35. SAIKAWA, K. The effect of rapid socio-economic development on the frequency of leprosy in a population. Lepr. Rev. 52 Suppl. (1981) 167s-l 75s.
36. STES, P, and MALATRE, X. Will the leprosy endemic in Rwanda soon be under control? Lepr. Rev. 60(1989) 139-146.
37. TONGLET, R., EECKHOUT, E., DEVERCHIN, J., BOLA, N., KIVITS, M. and PATTYN, S. Evaluation dti programme de lutte contre la lèpre dans les Uélés 0975-1989). Acta Leprol. 7 (1990) 145-152.
38. TONGLET, R.. PATTYN, S. R.. NSANSI, B. N.. EKCKHOUT, E. and DHVHRCHIN, J. The reduction of the leprosy endemicity in northeastern Zaire 1975/1989. Eur. J. Epidemiol. 6 (1990) 404-406.
39. VAN BEERS, S. M.. IZUMI, S.. MADJID, B., MALDA, Y., DAY, R. and KLATSER, R R. An epidemiological study of leprosy infection by serology and polymerase chain reaction. Int. J. Lepr. 62 ( 1994) 1-9.
40. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
41. WHO STUDY GROUP. Epidemiology of leprosy in relation to control. Geneva: World Health Organization. 1985. Tech. Rep. Ser. 716.
42. WORLD HEALTH ORGANIZATION. Progress towards the elimination of leprosy as a public health problem. Part I. Wkly. Epidemiol. Rec. 70 (1995) 177-182.
1. A. Meima, M.Sc., Department of Public Ilealth,Faculty of Medicine, Erasmus University Rotterdam, P. O. Box 173$, 3000 DR Rotterdam, The Netherlands.
2. M. D. Gupte, M.U., U.P.H., CJIL Field Unit, Inchain Council of Medical Rescarch, Avadi, Madras, India.
3. G.T. van Oortmarssen, Ph.D., Department of Public Health, Faculty of Medicine, Erasmus University, Rotterdam, The Netherlands.
4. J. D. F. Habbema, Ph.D., Department of Public Health, Faculty of Medicine, Erasmus University, Rotterdam, The Netherlands.
Reprint requests to A. Meima at the above address or FAX 31-10-435-6831.
Received for publication on 20 November 1996.
Accepted for publication in revised form on 31 March 1997.