- Volume 65 , Number 3
- Page: 320–7
A follow-up study of multibacillary hansen's disease patients treated with multidrug therapy (MDT) or MDT + immunotherapy (IMT)
ABSTRACT
Multibacillary (MB) leprosy patients treated with multidrug therapy (MDT) or MDT + immunotherapy (IMT) with BCG + heat-killed Mycobacterium leprae were tested annually for their ability to proliferate in vitro to the mycobacterial antigens BCG, M. leprae soluble extract, and intact M. leprae. IgM antibody responses to phenolic glycolipid I (PGL-I) were measured, as well as serum nitrite levels in patients' sera, before, during and after treatment. Patients who received only MDT did not present cellular reactivity to intact M. leprae antigens, in contrast to the results obtained with BCG, which elicited reactivity at time zero, that increased after treatment. Regarding PGL-I antibody variations in relation to the initial value, we observed a statistically significant marked decrease at the end of 2 years which continued to fall in successive evaluations. MB patients showed high initial serum nitrite concentrations which dropped drastically with treatment. This decay was apparently associated with the bacillary load present in these patients. The group submitted to IMT+MDT showed high and long-lasting T-cell responses to mycobacterial antigens in a significant number of initially unresponsive MB patients. There was a marked increase to M. leprae soluble extract and BCG, as well as a more variable response to whole bacilli. The antibody leveis in this group of patients are sustained for a somewhat longer period and decreased more slowly during the 5-year follow up.RÉSUMÉ
Des patients lépreux multibacillaires (MB) traités par polychimiothérapie (PCT) ou PCT + immunothérapie (IMT) avec BCG + Mycobacterium leprae tué par la chaleur ont été testés annuellement quant à leur capacité de produire une réaction de prolifération in vitro aux antigènes mycobactériens du BCG. d'un extrait soluble du M. leprae. et du M. leprae intact. Les réponses d'anticorps IgM au glycolipide phénolique I (PGL-I) ont été mesurées, ainsi que les taux sériques de nitrites chez. les patients avant, pendant et après le traitement. Les patients ayant reçu la seule PCT ne présentaient pas de réactivité cellulaire aux antigènes du M. leprae intact, EN contraste aux résultats obtenus avec le BCG, qui a provoqué une réactivité au temps zéro, et qui a augmenté après le traitement. EN ce qui concerne les variations d'anticorps vis-à-vis du PGL-I par rapport à leurs valeurs initiales, nous avons observé une diminution marquée statistiquement significative à la lin des deux ans, et qui a continué à décroître au cours des évaluations successives. Les patients MB ont montré des concentrations sériques initiales de nitrites élevées, qui ont fortement diminué avec le traitement. Cette diminution était apparemment associée à la charge bacillaire présente chez ces patients. Le groupe soumis à IMT+PCT a montré des réponses des cellules T importantes et de longue durée à des antigènes mycobactériens chez un nombre significatif de patients MB initialement non réactifs. Il y avait une augmentation marquée de la réactivité vis-àvis de l'extrait soluble de M. leprae et du BCG, ainsi qu'une réponse plus variable aux bacilles entiers. Les taux d'anticorps dans ce groupe de patients sont maintenus pour une période quelque peu plus longue et ont diminué plus lentement au cours des 5 ans de suivi.RESUMEN
Se trató un grupo de pacientes con poliquimioterapia (PQT) o con PQT + innunoterapia (IMT) con BCG + Mycobacterium leprae muerto por calor. Después, anualmente, los linfocitos de estos pacientes se probaron para medir su capacidad de proliferación in vitro en respuesta a su estimulación con BCG. con extracto soluble de M. leprae o con M. leprae intacto. También se midieron Las resptiestas IgM contra el glicolípido fenólico-I (PGL-I) y Los niveles de nitritos en el suero de los pacientes, antes, durante y después del tratamiento. Los pacientes que recibieron sólo PQT no presentaron reactividad celular contra los antígenos deM. leprae intacto, en contraste con los resultados obtenidos con BCD que indujeron una cierta reactividad en el tiempo cero, que aumentó después del tratamiento. En relación a los anticuerpos contra el PGL-I, observamos una marcada disminución en sus niveles que fare estadísticamente significativa al final de 2 años y que continuó decayendo en evaluaciones sucesivas. Los pacientes MB mostraron altos niveles iniciales de nitritos que decayeron drásticamente con el tratamiento. Esta disminución estuvo aparentemente asociada con la carga bacilar presente en estos pacientes.Un número significativo de los pacientes MB inicialmente no respondedores, que fueron sujetos a tratamiento con PQT+IMT, mostraron respuestas de células T elevadas y de larga duración hacia los antígenos micobacterianos. Globalmente hubo un marcado incremento en la respuesta a los antígenos del extracto soluble de M. leprae y al BCG, asi como una respuesta más variable contra el bacilo completo. Los niveles de anticuerpos en este grupo de pacientes se mantuvieron elevados durante un periodo mayor de tiempo y luego disminuyeron lentamente durante los 5 años de seguimiento.Hansen's disease presents a broad spectrum of clinical and histopathological manifestations which reflect the nature of the individual's immune response to Mycobacterium leprae. This spectrum of clinical manifestations includes two polar types of infection, lepromatous leprosy (LL) and tuberculoid leprosy (TT), as well as intermediate borderline forms of disease (17). To simplify field work, the World Health Organization (WHO) classifies leprosy patients according to the bacillary load as multibacillary (MB) patients, with an elevated bacterial load, and paucibacillary (PB) patients, who present negative or weak bacilloscopy.
Cell-mediated immunity (CMI) plays a major role in resistance and protection to intracellular infection. In leprosy, as in other intracellular infections, CMI depends on the ability to develop an effective T-cellmediated immune response against the microorganism. This cellular reactivity is low or absent in MB patients; negativity persists for many years after chemotherapy in most patients.
Tissues infected with M. leprae contain large amounts of phenolic glycolipid-I (PGL-I), which is a highly specific antigen of the microorganism. Antibody levels against PGL-I are frequently used to follow up the therapeutic response and elimination of the bacillary load in patients under treatment (2, 6, 13).
M. leprae is an obligate intracellular pathogen that invades and multiplies within monocytes/macrophages, activating the metabolic burst and the production of toxic radicals. High levels of nitrite in serum have been associated with several pathologies (19, 20) and provide an indirect measurement of nitric oxide (NO) production by endothelial cells, activated macrophages and neurons (9, 11).
Because of bacterial resistance induced by monodrug therapy, it has been necessary to develop and implement effective multidrug treatment of leprosy patients (1, 7, 23). In the Institute of Biomedicine, Caracas, Venezuela, MB leprosy patients are treated with multidrug therapy (MDT), with or without simultaneous immunotherapy (IMT) with a combined vaccine containing heatkilled M. leprae plus BCG. An effort is made to systematically follow the two groups for a period of 5 years or more, with repeated clinical, histopathological and immunological evaluations.
In a previous study, we evaluated the immune responses of patients submitted to IMT+MDT before treatment and after a 5year follow up (16). In our present study, we report an annual follow up for at least 5 years of CMI and levels of serum antibody to PGL-I in MB patients treated with MDT alone as well as with MDT+IMT. The preliminary results of the determination of serum nitrite levels in both MB and PB patients are also reported.
MATERIALS AND METHODS
Patients and treatments. All patients were adults and were classified according to WHO criteria. MDT with dapsone (DDS), rifampin and clofazimine was administered using WHO protocols (24). IMT, as described previously by Convit, et al. (7), was administered together with MDT in a group of 88 MB patients (55 males, 33 females). Briefly, IMT consisted of 10 doses of vaccine containing 6 x 108 heat-killed M. leprae plus variable concentrations of BCG (0.01 to 0.2 mg, depending on previous reactivity to PPD), administered intradermally during a 2-year period. A second group of 74 MB patients (48 males, 26 females) received MDT alone. Samples were taken before treatment (time - 0) and from as many patients as possible, as indicated in the tables and figures, at regular intervals until therapy was terminated and for several years after therapy; inevitably follow up was difficult since the majority of the patients live in rural areas of Venezuela and constitute a mobile population. The mean bacterial index (BI) before starting treatment in both MB groups was 4+, and they were all Mitsuda lepromin skin-test negative (<6 mm).
The nirite levels in sera from 12 MB patients (9 males, 3 females) and 12 PB patients (10 males, 2 females) were determined. The PB patients received MDT; the MB group includes patients treated with MDT and MDT+IMT, since preliminary analysis showed no differences between the two forms of therapy.
Lymphocyte transformation test (LTT). Mononuclear cells were obtained from 20 ml of heparinized blood as described by Boyum (3) and processed with the antigens listed below. The stimulation index (SI) was calculated by dividing the mean counts per minute (cpm) in duplicate antigen-containing cultures by the mean cpm in antigen-free cell cultures. The percentages of the positive tests with an SI of >2 are reported.
Antigens. In the LTT tests, heat-killed BCG (Connaught Laboratories, Willowdale, Ontario, Canada) was employed at a concentration of 3.6 µ g/200 µ l Intact M. leprae (BP) and soluble antigens (M1SA) were obtained by previously described protocols (16), and were used at concentrations of 1.2 x 106 bacilli/200 µ l and 5 µ g/200 µ l, respectively.
Serologic assay. IgM antibodies against PGL-I were determined in an enzymelinked immunosorbent assay (ELISA) performed as described previously (21). Native PGL-I purified from M. leprae -infected armadillo liver was kindly supplied by Dr. Patrick Brennan, Colorado State University, Fort Collins, Colorado, U.S.A. For each patient, the values were expressed as a percentage of change with respect to the first value, using the equation vk-vo/vo. Vo equals the value of anti-PGL-I antibody (optical density, OD) at time 0 (before treatment), and vk indicates antibody levels in successive years; an average for each year during the follow up was calculated. This formula was used for a more precise analysis because of the differences in the initial value of each patient.
Nitrite determination. Nitrite levels were measured in sera using the Griess reaction (8). Briefly, 50 µ l of serum was mixed with 150 µ l of Griess reagent, incubated for 10 min at room temperature, and the absorbance measured at 570 nm. The amount of nitrite was determined using a sodium nitrite standard curve from 2 to 50 µ M. All experiments were performed in triplicate.
Statistics. The serological data were expressed as annual changes and the statistical significance was determined by Student's t test. The chi-squared test was used for the LTT data.
RESULTS
LTT assay. Figure 1 (A, B, C) shows the in vitro evolution of cellular proliferation to BCG, M1SA and BP in MB patients. In patients treated with IMT+MDT, we observed a progressive increase in CMI to different antigens during the course of the study. BCG gave the highest response, varying from about 40% before treatment to 72% at the end of treatment (2 years later); it remained about 80% until the end of the study (Fig. 1A). The proliferation percentage with M1SA increases to about 40% in the first year after initiating treatment and this value is maintained throughout the study (Fig. 1B). The response to BP was low and variable as compared to BCG and M1SA (Fig. 1C).
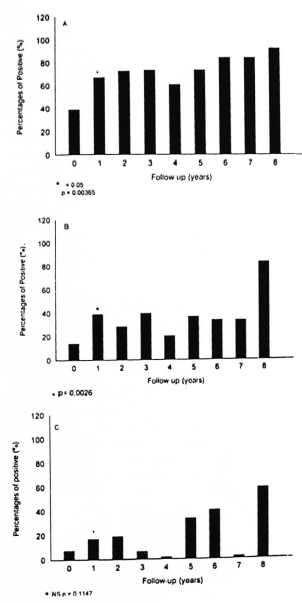
Fig. 1. Percentage of positive LTT reactions to BCG (A), M. leprae soluble extract (MISA) (B), and intact bacilli (BP) (C) in multibacillary patients treated with IMT+MDT. Number of patients at each yearly interval: Year 0 = 75, I = 42, 2 = 32, 3 = 33, 4 = 20, 5= 12,6= 11,7 = 6,8 = 6.
In MB patients treated with MDT only, we could not detect specific T-cell proliferation in response to BP. We detected some initial reactivity to M1SA in MB patients that were treated with MDT only which did not persist during the course of the study (Table 1). The persistence of the response to BCG during the entire time of evaluation was highly significant.
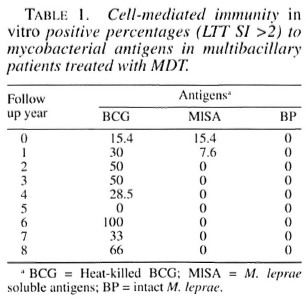
Serologic assay. The outcome of the 5year study of MB patients treated with MDT and IMT+MDT is shown in Figure 2 (A and B). Patients who received only MDT treatment showed a significant decrease in antibody levels during the first 2 years (Fig. 2A). The level of antibodies against PGL-I dropped by 57% in MDT patients after 2 years of treatment, in marked contrast with the group of patients receiving MDT+IMT whose antibody levels decreased by only 26.1% (Fig. 2B). The values in the two groups were similar at 3 years. Mean ELISA values throughout the course of the study are shown in Table 2. In this simpler analysis, the progressive decline in both groups is evident, although somewhat slower in patients receiving MDT+IMT.
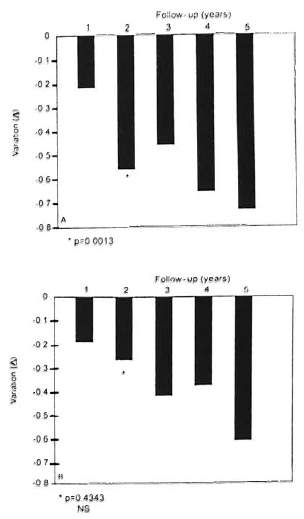
Fig. 2. ELISA variation (changes) in multibacillary patients treated with MDT (A) or MDT+ IMT (B).
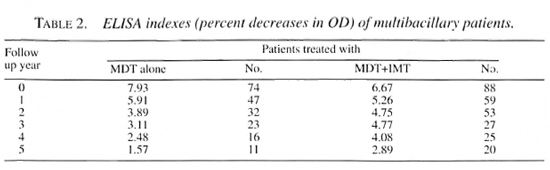
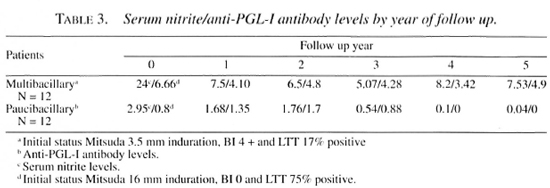
Nitrite determination. As shown in Figure 3, sera from untreated MB patients present a large amount of nitrite (about 25 µ M). This value dropped as low as that of a small group of healthy contacts in 2 years. In contrast, sera from PB patients gave nitrite concentrations somewhat lower than controls. The differences were not statistically significant and did not change over time. The differences in the initial Mitsuda reactivity, BI and LTT as well as yearly anti-PGL-I means in the two patient groups are shown in Table 3.
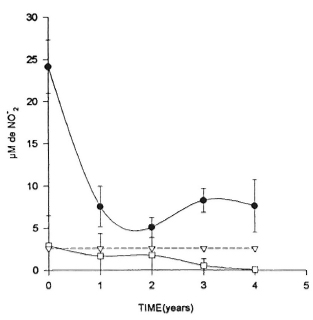
Fig. 3. Serrim nitrite concentration in MB (•) and PB ( ) patients anca hcalthy contacts (
) patients anca hcalthy contacts ( ) in a 5-year follow up.
) in a 5-year follow up.
DISCUSSION
Our observations confirm that CMI to M. leprae is induced when initially unresponsive MB patients are treated with IMT and MDT and demonstrate persistence of reactivity for at least 8 years (LTT data). In patients submitted to IMT+MDT, the highest response of T-cell proliferation was obtained using BCG antigen, followed by M1SA and, finally, BP. In an earlier study, we reported a substantially higher response to intact bacilli in Mitsuda-positive contacts and tuberculoid patients (77% and 51 %, respectively) (15). This difference may reflect differences between the normal induction of CMI by natural infection or treatment with IMT in severely compromised patients, or it may REFLECT differences among different lots of bacilli.
The normal response to BCG may provide a highly localized source of antigenpresenting cells, cytokines, adhesion molecules and other factors which bypass the immunological defects in nonrcactors to M. leprae. Healthy volunteers, who had been vaccinated with BCG during childhood, when vaccinated with killed M. leprae showed a persistently high proliferative response to M. leprae, suggesting that killed M. leprae has strong immunopotentiating activity (14). Interestingly enough, background response levels to BCG are higher in Venezuela than in a nonendemic British population (18).
Earlier studies have reported that patients treated with MDT and IMT showed clinical and histopathological changes as well as positive intradermal reactions to PPD, lepromin and soluble antigens OF M. leprae (7, 16).
Recently, Majumder, et al. reported clinical cure OFLEPROMATOUS patients using a low dose OF Convit vaccine along with MDT in a Calcutta trial They found a more rapid decrease of bacterial and morphological indexes in patients receiving vaccine + MDT than in patients who were given BCG + MDT or only MDT. These interesting parameters are not reported in the present study because of our incomplete data base.
MB patients treated with MDT did not acquire positive reactivity to M. leprae antigens during this prolonged follow up, confirming many earlier observations. Lymphocyte proliferation in assays with M. leprae antigens was generally negative during the follow up, although low initial and 1year reactivity to soluble extract was observed. However, the response to BCG antigen was maintained during the study. The number of individuals studied decreased over time, and this could explain the variations reported.
During the period of this study, confirmed relapses or re-infections have not been observed in either of the study groups. Occasional inflammatory skin lesions have been observed after therapy was completed but intact bacilli could not be detected, and these lesions appear to correspond to delayed reactions to persisting bacterial debris.
Measurement of antibodies against PGL-I is used as a possible tool for the detection of new cases, classification of patients within the immune spectrum, and evaluation of the therapeutic response (4, 6, 13, 21, 25). We observed a progressive decrease of antibodies to PGL-I in both groups during the course of the study. The fact that PGL-I is not water soluble and its degradation is slow, remaining for a long period of time in the absence of viable bacilli (13), undoubtedly contributes to a relatively slow decline in antibody levels.
It is rather surprising that the decline was slower in the group treated with IMT+MDT than in the group treated with MDT alone. Study of larger groups would be helpful to confirm this observation. Nevertheless, we might speculate that the presence of a population of specifically sensitized T cells may contribute to the persistence of significant levels of antibodies to even very small amounts of bacterial antigen. These sensitized T cells collaborate in antibody synthesis by B cells, even to nonprotein antigens, through the presence of T-cell receptors which do not bind to the more frequent peptide-major histocompatibility complex molecules usually associated with T-cell responses. Frequent reports of IgG antibodies to PGL-I apparently confirm such mechanisms, and the study of the relative proportion of IgM and IgG antibodies in untreated patients and patients treated with MDT alone or IMT+MDT would be of considerable interest.
The nitrite data suggest that effector cells, possibly macrophages, were activated to produce high levels of NO, which is known as a potent effector molecule involved in the killing of a variety of bacteria (5) and parasites (22). It has been reported that the bacillary load is reduced during the first 6 months of MDT (6). Therefore, the decline in nitrite concentration after treatment may be proportional to the elimination of the microorganisms. In preliminary studies, we saw no differences between groups treated with MDT or MDT+IMT.
Since MB patients do not possess effective CM I mechanisms, we cannot attribute the high initial nitrite levels to conventional immunological mechanisms induced by activated lymphocytes. A much more detailed study is required to confirm these preliminary observations and to evaluate their significance. Since serum nitrite levels may originate from diverse cells, the presence of high concentrations may be entirely unrelated to macrophage activation, or may reflect the requirement for additional immunologically activated mediators for effective killing.
We consider that the goals of leprosy treatment should include not only the relatively rapid eradication of the vast majority of viable bacilli with efficient supervised drug therapy, but also the induction of Tcell reactivity and long-lasting memory to protect patients from reinfection or relapse. Because of the extraodinarily long latent period of the development of leprosy in immunologically compromised patients, the detection of relapse will require follow up of very large numbers of patients over many years, but these data will eventually contribute to our knowledge about the treatment strategies required not only for control as a public health problem, but also the eventual eradication of the disease. The development of new strategies toward the design of second-generation vaccines based on the use of specific epitopes with a demonstrable protective effect remains as a longterm goal for immunotherapy, prophylaxis and eventual eradication.
Acknowledgment. The authors thank all of the technical personnel and public health inspectors who collaborated in this study. We also thank Dr. Rafael Borges from the Epidemiology Section for the statistical analyses and Wilman Clark for preparation of the ligures.
REFERENCES
1. ARANZAZU, N. and ACOSTA, L. Enfermedad de Hansen. In: Dermatología Rondón Lugo. Godoy, R., ed. Caracas: Industria Editorial Venezolana, 1995, pp. 557-569.
2. BACH, M. A., W ALLACH, D., FLAGEUL, B., HOFEENBACH, A. and COTTENOT, F. Antibodies to phenolic glycolipid-1 and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lep. 54 (1986) 256-267.
3. BOYUM, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21 (1968) 77-89.
4. BRENNAN, P. J. and BARROW, W. W. Evidence for species-specific lipid antigens in M. leprae. Int. J. Lepr. 48(1980) 382-387.'
5. BUSTER, B. L., WETROB, A. C, TOWNSEND, G. C. and SCHELD, W. M. Potential role of nitric oxide in the pathophysiology of experimental bacterial meningitis in rats. Infect. Immun. 63 (1995) 3835-3839.
6. CHANTEAU, S., CARTEL, I. L., CELERIER, P., PLICHART, R., DESFORGES, S. and ROUX, J. PGL-I antigen and antibodies detection in leprosy patients: evolution under chemotherapy. Int. J. Lepr. 57(1989) 735-743.
7. CONVIT, J., ULRICH, M., A RANZAZU, N., CASTELLANOS, P. L., PINARDI. M. E. and REYES, O. The development of a vaccination model using two microorganisms and its applications in leprosy and leishmaniasis. Lepr. Rev. 57 (1986) 263-273.
8. GREEN, L. C. WAGNER, D. A., GLOGOWSKI, J., SKIPPER, P. L., WISHNOK, J. S. and T ANNENBAUM, S. R. Analysis of nitrate, nitrite and (15 N) nitrate in biological fluids. Anal. Biochem. 126 (1982) 131-138.
9. HIBBS, J. B., JR., TAINDOR, R. and VAVRIN, Z. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 157(1988)87-94.
10. JI, B. and GROSSET, J. II. Recent advances in the chemotherapy of leprosy. Lepr. Rev. 61 (1990) 313-329.
11. KNOWELS, R. G., PALACIOS, R., PALMER, M. J. and MONCADA, S. Formation of nitric oxide from Larginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 86(1989)5159-5162.
12. MAJUMDER, V, MUKERJEE, A., HAJRA, S. K., SAHA, B. and SAHA, K. Immunotherapy of far-advanced lepromatous leprosy patients with lowdose Convit vaccine along with multidrug therapy (Calcutta trial). Int. J. Lepr. 64 (1996) 26-36.
13. MEEKER, H. C, SHULLER-LEVIS, G., FUSCO, F, GIARDINA-BECKET, M., SERSEN, E. and LEVIS, W. Sequential monitoring of leprosy patients with serum antibody levels to phenolic glycolipid-l and mycobacterial lipoarabinomannan. Int. J. Lepr. 58 (1990) 503-511.
14. MUSTAFA, A. S. and OFTUNG, F. Long lasting T cell reactivity to Mycobacterium leprae antigens in human volunteers vaccinated with killed M. leprae. Vaccine 11 (1993) 1108-1112.
15. RADA, E., SANTAELI.A, C, ARANZAZU, N. and CONVIT, J. Preliminary study of cellular immunity to Mycobacterium leprae protein in contacts and leprosy patients. Int. J. Lepr. 60 (1992) 189-194.
16. RADA, E., ULRICH, M., ARANZAZU, N., SANTAELI.A, C, GALLINOTO, M. E., CENTENO, M., RODRIGUEZ, V. and CONVIT, J. A longitudinal study of immunologic reactivity in leprosy patients treated with immunotherapy. Int. J. Lepr. 62 (1994) 552-558.
17. RIDI EY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system system. Int. J. Lepr. 34 (1966) 255-273.
18. SHARPLES, C. E., SHAW, M., CASTES, M.. CONVIT, J. and BLACKWELL, J. Immune response in healthy volunteers vaccinated with BCG plus killed leishmanial promastigotes antibody responses to mycobacterial and leishmanial antigens. Vaccine 12 (1994) 1402-1412.
19. SHIRAISHl, T., DEMEESTER, S. R., W ORRALL, N. K., RITTER, J. H., MISKO, T. P., FERGUNSON, T. B., JR., COOPER, J. D. and PATTERSON, G. A. Inhibition of inducible nitric oxide synthetase ameliorates rat lung allograft rejection. J. Thorac. Cardiovasc. Surg. 110(1995) 1449-1459.
20. THAI, S. F., LEWIS, J. B., W ILLIAMS, R. B., JOHNSON, S. P. and A DAMS D. O . Effect of oxidized 65, 3 Rada, et al.: Follow Up: LDL on mononuclear phagocytes: inhibition of induction of four inflammatory cytokine gene RNAs, release of NO, and cytolysis of tumor cells. Leukoc. Biol. 57 (1995) 427-433.
21. ULRICTI, M , SMITH, P. G., SAMPSON, C, ZUNIGA, M., CENTENO, M., GARCIA, V., MANRIQUE, X., SALGADO, A. and CONVIT, J. IgM antibodies to native phenolic glycolipid-1 in contacts of leprosy patients in Venezuela: epidemiological observations and prospective study of the risk of leprosy. Int. J. Lepr. 59 (1991) 405-415.
22. VOULDOUKIS, I., RIVEROS-MORENO, V, DUGAS, B., OUAAZ, F., BECHEREL, P., DEBRE, P., MONCADA, S. and MOSSAI.VLAYI, D. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fce(RII/CD23) surface antigens. Proc. Natl. Acad. Sci U.S.A. 92 (1995) 7804-7808.
23. WATERS, M. F. R. Chemotherapy of leprosy- current status and future prospects. Trans. R. Soc. Trop. Med. Hyg. 87 (1993) 500-503.
24. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768
25. YOUNG, D. B. and BUCHANAN, T. H. A serological test for leprosy with a glycolipid specific for M. leprae. Science 221 (1983) 1057-1059.
1. E. Rada, M.S. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
2. M. Ulrich, Ph.D. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
3. N. Aranzazu M.D. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
4. M.D.; V. Rodrieuez, M.S. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
5. M. Centeno. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
6. I. Gonzalez, M.S. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
7. C. Santaella, M.S. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
8. M. Rodriguez, Ph.D. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
9. J. Convit, M.D. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
Received for publication on 9 September 1996.
Accepted for publication in revised form on 17 March 1997.