- Volume 65 , Number 3
- Page: 366–71
Disseminated intravascular coagulopathy as an adverse reaction to intermittent rifampin schedule in the treatment of leprosy
In an effort to increase the utility of the Journal in continuing medical education, in this section we welcome contributions dealing with practical problems in leprosy work. Submissions to this section will undergo minimal editorial changes and may well contain controversial points. Letters to the Editor pointing out other viewpoints are welcome.
Rifampin has proved to be effective for the treatment of leprosy,1 and multicenter clinical studies 2'1 have demonstrated its efficacy when administered in intermittent or daily doses. Thus, rifampin has been indicated for treatment in monthly doses as part of the multiple drug therapy program of leprosy control proposed by the World Health Organization (WHO/MDT) in 1981,4 which consists of the following different regimens: 100 mg dapsone self-administered daily and 600 mg of monthly supervised rifampin for 6 months for paucibacillary (PB) patients, and 100 mg dapsone and 50 mg clofazimine self-administered daily and 600 mg of monthly supervised rifampin in combination with 300 mg clofamizine for 24 months for multibacillary (MB) patients.
Reports of peculiar adverse reactions to rifampin have been published since the late 1960s when the drug was administered intermittently in an attempt to treat tuberculosis.5-7 The adverse reactions observed with daily or intermittent doses were skin rashes (<5%), gastro-intestinal disorders (variable), hepatitis (<1%), and rarely purpura. Reactions observed only after intermittent administration were a flu-like syndrome and rare, potentially serious, effects requiring immediate discontinuation of treatment and admission of patients for immediate treatment such as shock, respiratory disorders (shortness of breath), hematologic alterations (hemolysis and thrombocytopenia), renal insufficiency, and pancreatitis.7, 8 Considering the efficacy and cost of rifampin and its possible toxic effects observed after its intermittent use, but not in clinical studies using the drug for the treatment of leprosy,2, 3, 9 the WHO recommended a program of leprosy control using monthly doses of rifampin under supervised administration in order to facilitate the detection of possible adverse effects.
Since the end of the 1970s, the National Division for Sanitary Dermatology and the Ministry of Health (NDSD/MH) of Brazil have indicated the association of rifampin, 600 mg/day for 3 months, and dapsone, 100 mg/day, with curative objectives for borderline and lepromatous leprosy patients, while PB patients should receive only a 2-year course of daily dapsone.10 However, in 1991 the WHO/MDT was officially adopted in Brazil for all new cases and also for those who had not completed 5 years of treatment or who had clinical signs of active disease, or with a positive bacilloscopy.10
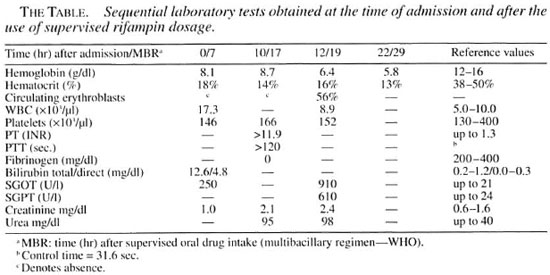
We report here the occurrence of disseminated intravascular coagulation (DIC) and hemorrhagic liver necrosis due to the monthly use of rifampin at the recommended dose for leprosy treatment (600 mg). A 46-year-old female patient with lepromatous leprosy, with a 3.0 bacterial index (BI), presented with multiple nodules on the alae nasi, earlobes, upper limbs including the hands, lower limbs and trunk, together with madarosis, erythema and infiltration of the face and of extensive areas of the tegument. The patient was treated with sulfone (100 mg/day) for 6 years, in combination with rifampin (600 mg/day) for the first 3 months of treatment, according to the NDSD/MH regimen, and showed good progress, disinfiltration of the involved areas, flattening of the lesions and a reduction of the BI without relevant adverse effects. She then began a multiple drug therapy regimen for MB patients (WHO/MDT) and tolerated well the first two supervised doses, with no explicit complaints. However, I hr after her third supervised dose she presented with intense headache, pain in the precordial, epigastric and lumbar regions associated with hemateniesis and macroscopic hematuria. Examination upon admission to the hospital revealed an agitated patient with profuse sweating, pallor, cold extremities, tachycardia (120 bpm), and arterial hypertension (180/110 mm Hg). Seven hours alter the beginning of these symptoms, the pain lessened and the arterial pressure and heart rate tended to stabilize (130/90 mm Hg and 92 bpm. respectively). Jaundice was observed in addition to the initial clinical signs and symptoms, and laboratory tests showed intense bilirubinemia (mainly unconjugated bilirubin) and anemia, with normal renal function (The Table). Digestive hemorrhage was confirmed and gastroscopy revealed multiple hemorrhagic sites, especially upon endoscopic trauma. The patient progressed in an unstable manner and 12 hr after admission her general condition worsened, with marked dehydration, moderate pallor, dyspnea, evident jaundice, cyanosis of lips and extremities, tachycardia (120 bpm), ecchymoses of the upper limbs and spontaneous gingival and genital bleeding. Laboratory tests revealed a marked fall in hemoglobin level (Hb = 6.4 g/dl) and discrete creatinine and urea elevation. A clinical diagnosis of disseminated intravascular coagulation was later confirmed by significant changes in prothrombin time (PT), activated partial thromboplastin time (APTT), with a very low fibrinogen level (The Table). Therapeutic measures (fresh plasma, cryoprecipitate. heparin, corticotherapy) did not prevent the progressive deterioration of the general condition and neurological impairment of the patient, with respiratory insufficiency and cardiac arrest culminating in death 22 hr after admission to the hospital. The autopsy report revealed hemorrhagic hepatic necrosis with histological features compatible with sensitization to the drug.
DISCUSSION
The use of rifampin has been related to hematologic alterations, the most frequent being hemolytic anemia,14-23 purpura associated or not with thrombocytopenia, and asymptomatic thrombocytopenia.5, 7, 12, 13 Acute hemolysis of discrete-to-massive in tensity has been reported to occur14, 23 after the intermittent irregular use of rifampin or even after reintroduction of rifampin followed by a period without daily administration that may vary from a few days to several years.19 A few cases of disseminated intravascular coagulation related to the use of rifampin have been documented.17, 23 Mattson7 subdivided systemic reactions into five symptom complexes: 1) flu-like syndrome (tremors, fever, muscle pains, headache, malaise, and joint edema); 2) abdominal syndrome (abdominal pain, nausea, diarrhea and vomiting); 3) cutaneous syndrome (Hushing and itching with or without a cutaneous rash, especially on the face and neck); 4) respiratory syndrome (dyspnea with bronchospasm); 5) hematologic syndrome (laboratory abnormalities of blood components and clinical correlation). Characteristically, the syndromes initiate 2-4 hr after drug ingestion and may occur simultaneously or in sequence, with a flu-like syndrome alone or in combination with other syndromes being the most frequent presentation. It has been demonstrated in clinical assays that the flu-like syndrome was associated with an increase in plasma-free hemoglobin, reticulocytes, and bilirubins and with a gradual decrease in blood hemoglobin and hematocrit.16 Hemolysis associated with a flu-like syndrome is also supported by the increase in reticulocytes in sequential analyses, suggesting that this reaction may represent the first sign of intravascular hemolysis, differing only in quantitative terms from more serious reactions,16 pointing out that the flu-like syndrome should be considered potentially hazardous.
Adverse effects have been described with monthly rifampin administration (600 mg) for the treatment of leprosy, including the flu-like syndrome,24, 25, 35 acute renal failure,20, 26, 27, 28, 29, 34 shortness of breath and urticaria.30 intravascular hemolysis,20, 31 thrombocytopenia12, 20, 32, 34 and shock.33
In the present case there was strong evidence of massive hemolysis progressing to DIC, as suggested by the clinical data and confirmed by altered laboratory tests (PT, APTT and low fibrinogen), temporally related to the use of a monthly dose of rifampin. Hepatic involvement might be due to the massive immune hemolysis added to aggression by the drug, leading to acute impairment of the hepatic production of coagulation proteins and worsening the imminent DIC. A direct relationship between intermittent doses (some studies with high doses) of rifampin and the increased frequency of peculiar side effects is frequently
Opinions about the mechanisms causing the adverse reactions attributed to rifampin are divergent, and it has been suggested that the nature of the systemic reaction is more immune than toxic." It is postulated that rifampin is a potentially immunogenic molecule because of its ability to participate in lipophilic reactions with circulating free proteins (albumin, gamma-globulin, etc.) or with proteins associated with the cell membrane, resulting in an antigen-carrier complex with potential immunogenicity.39 The formation of immune complexes from protein-bound rifampin, plus anti-rifampin antibodies and complement activation, may trigger the clinical syndrome. In a study of the affinity of the drug for red blood cells, Dukor, et al.39 detected rifampin bound to the cell membrane. However, it has also been proposed that red blood cells and platelets may be simply "innocent bystanders" due to the nonspecific interactions of rifampin with blood cells.22, 39 Anti-rifampin antibodies can be detected in patients using rifampin and are more frequently related to adverse reactions,6, 14, 17, 21, 22, 40 suggesting the potential role of the antigen-antibody complex, although these complexes have not been demonstrated in all patients.5, 6, 13 Interpretation of the data is difficult because of the variable results obtained by the different techniques for antibody detection and, perhaps more importantly, because there is demonstrated nonspecific interaction of rifampin with gamma-globulin,39 among other serum proteins, a fact that may simulate specificity.
In the case reported here the hemolytic reaction that developed from antibody formation was strongly suggested by the positivity of the direct antiglobulin test associated with a progressive fall in hemoglobin levels and the more pronounced increase in unconjugated bilirubin. Intermittent administration of certain antigens is particularly appropriate for the induction of antibodies. Indeed, sensitization may occur more frequently in patients receiving rifampin intermittently or irregularly rather than at daily doses. The present data suggest that sensitization occurred during the initial exposure to rifampin in the first therapeutic regimen administered to the patient and that re-exposure to an intermittent rifampin schedule triggered the fatal outcome.
In the case reported here the patient was treated with dapsone in combination with rifampin for 3 months and dapsone alone for 6 years, with no adverse effects, a fact suggesting that dapsone as part of the WHO/MDT for MB patients was not causally related to the adverse outcome. Furthermore, we identified similar cases of DIC in the literature17, 23 which involved patients using a therapeutic schedule for tuberculosis. The temporal association of severe side effects with rifampin use was then unequivocal.7, 8 Also, early diagnosis of the systemic adverse reaction resulting in rapid interruption of rifampin reduces the severity of the adverse effects, permitting the safe continuation of treatment with dapsone and clofazimine in combination with a rifampin substitute.3, 4
The suspected occurrence of adverse reactions, including death, related to the implementation of WHO/MDT in the state of São Paulo, Brazil, has led to the organization of a surveillance approach, and the collected data have been reported by Brasil, et al.34 Adverse effects were evaluated in 20,667 patients treated with WHO/MDT from 1991 to 1993. Of these, 7020 cases were newly diagnosed and 13,647 had received previous treatment. There were 127 notifications of adverse effects subdivided as follows: a) flu-like syndrome (54 cases), b) acute renal failure (20 cases), c) thrombocytopenic purpura (2 eases), and d) disseminated intravascular coagulation (present ease). Adverse effects were 7.3/1000 for MB patients treated with WHO/MDT after being previously treated with daily rifampin for 3 months as in the national program (NDSD/MH). These patients were more severely involved, with 73.3% having a flu-like syndrome or acute renal failure.
The WHO/MDT is the best therapeutic option available at present for the treatment of leprosy and has had an unequivocal impact on the reduction of its prevalence with the ability to provide a cure. However, it should be pointed out that patients receiving intermittent treatment with rifampin for the control of leprosy need appropriate supervision, as recommended by the WHO.4 Although adverse reactions are rare, health professionals should be prepared to anticipate them. Patients submitted to continuous long-lasting treatment who re-initiate the use of rifampin after previous exposure are considered to be at risk for adverse effects. Thus, any systemic symptom should be an alert for the appropriate investigation and evaluation of the discontinuation of rifampin.
1 Rees, R. J. W., Pearson, J.M.H. and Waters, M. F. R. Experimental and clinical studies on rifampicin in treatment of leprosy. Br. Med. J. 1 (1970) 89-92.
2 Oppromolla, D. V., Tonello, C. J. S., McDougall, A. C. and Yawalkar, S. J. A controlled trial to compare the therapeutic effects of dapsone in combination with daily or once-monthly rifampicin in patients with lepromatous leprosy. Int. J. Lepr. 49 (1981) 393-397.
3 Languillon, J., Yawalkar, S. J. and McDougall, A. C. Therapeutic effects of adding Rimactane® (rifampin) 450 milligrams daily or 1200 milligrams once monthly in a single dose to dapsone 50 milligrams daily in patients with lepromatous leprosy. Int. J. Lepr. 47 (1979)37-43.
4 WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
5 Poole, G., Stradling, P. and Worlledge, S. Potentially serious side effects of high-dose twice-weekly rifampicin. Br. Med. J. 3 (1971) 343-347.
6 Worlledge, S. The detection of rifampicin-dependent antibodies. Scand. J. Respir. Dis. Suppl. 84 (1973) 60-63.
7 Mattson, K. Side effects of rifampicin-a clinical study. Scand. J. Respir. Dis. Suppl. 82 (1973) 1-52.
8 Girling, D. J. and Hitze, K. H. Adverse reactions to rifampicin. Bull. WHO 57 (1979) 45-49.
9 Rees, R. J. W. Rifampicin: the investigation of a bactericidal antileprosy drug. Lepr. Rev. Suppl. 46 (1975)121-124.
10 Brasil Ministério da Saúde. Fundação Nacional de Saúde. Normas técnicas e procedimentos pare utilização dos esquemas de poliquimioterapia no tratamento da hanseníase. Brasília: Ministério da Saúde, 1990.
11 Wilawska-Orlovvska, B. and Pniewski, T, Haematological changes during intermittent treatment with rifampicin. Scand. J. Respir. Dis. Suppl. 84 (1973) 94-97.
12 Nishioka, S. A., Goulart, I. M. B., Nunes-Araujo, F. R. F, Arantes, S. C. F., Burgarelli, M. K. N., Ferreira, M. S., Santos, R. P. and Lopes, V. R. Severe thrombocytopenia and intermittent use to rifampin. Int. J. Lepr. 60(1992) 273-274.
13 Stradling, P. Side effects observed during intermittent rifampicin therapy. Scand. J. Respir. Dis. Suppl. 84 (1973) 129-131.
14 Lakshminarayan, S., Satin, S. A. and Hudson, L. D. Massive haemolysis caused by rifampicin. Br. Med. J. 2 (1973) 282-283.
15 Criei, A. and Verwilghen, R. L. Intravascular haemolysis and renal failure caused by intermittent rifampicin treatment. Blut 40 (1980) 147-150.
16 Mattson, K. and Janne, J. Mild intravasal haemolysis associated with flu-syndrome during intermittent rifampicin treatment. Eur. J. Respir. Dis. 63 (1982) 68-72.
17 Denis, J., Robert, A., Johanet, C., Homberg, J. C., Opolon, P. and Levy, V. G. Accident immunoallergique à la rifampicine avec coagulation intravasculaire disséminée. Presse Méd. 12 0983) 1479-1481.
18 Tahan, S. R., Diamond, J. R., Blank. J. M. and Horan, R. F. Acute hemolysis and renal failure with rifampicin-dependent antibodies after discontinuous administration. Transfusion 25 (1985) 124-127.
19 Assenfeld, A. H. W. Renal failure and haemolysis caused by rifampicin. Tubercle 67 (1986) 234-235.
20 Papaiordanou, P. M. O., Branching M. L. M., Gonçales Junir, F. L, Aoki, F. L, Boccato, R. S. B. S., Ramos, M. C. and Pedro, R. J. Efeito adverso do uso intermitente de Rifampicina para tratamento da Hanseníase. Rev. Inst. Med. Trop. São Paulo 30 (1988) 383-386.
21 Govoni, M., Moretti, M., Menini, C. and Fioochi, F. Rifampicin-induced immune hemolytic anemia: therapeutic relevance of plasma exchange. Vox Sang. 59(1990)246-247.
22 Pereira, A., Sanz, C., Cervantes, F. and Castillo, R. Immune hemolytic anemia and renal failure associated with rifampicin-dependent antibodies with anti-I specificity. Ann. Hematol. 63 (1991) 56-58.
23 Ip, M., Cheng, K. P. and Cheung, W.C. Disseminated intravascular coagulopathy associated with rifampicin. Tubercle 72 ( 1991) 291-293.
24 Vaz, M., Jacob, A. J. and Rajendran, A. "Flu" syndrome on once monthly rifampicin: a case report. Lepr. Rev. 60 (1989) 300-302.
25 Dhar, S., Kaur, I., Sharma. V. K. and Kumar, B. "Flu" syndrome due to rifampin: experience with four cases. Int. J. Lepr. 63 (1995) 92-94.
26 Kar, H. K. and Roy, R. G. Reversible acute renal failure due to monthly administration of rifampin in a leprosy patient. Indian J. Lepr. 56 (1984) 835-839.
27 Dedhia, N. M., Almeida A. F., Khanna, U. B., Mittal, B. V. and Acharya, V. M. Acute renal failure: a complication of new multidrug regimen for treatment of leprosy. Int. J. Lepr. 54 (1986) 380-328.
28 Cusumano, A., Caldentey, D., Marise, C, Raimondo, M. and Ibarra, R. Renal cortical necrosis as complication of leprosy treatment. (Letter) Clin. Nephrol. 38 (1992): 172-173.
29 Gordan, P. A., Grion, C. M. C., Sousa. V, Carvalho, V. P., Delfino. V. D. A., Mendes, M. F., Matini, A. M. and Mocelini, A. J. lnsuficiencia renal aguda pelo uso do esquema multidroga na hansenfase. Hansenol. Int. 17(1992)21-26.
30 Gupta, C. M. and Bhate, R. D. Shortness of breath with urticaria due to once monthly rifampicin. (Letter) Lepr. Rev. 58 (1987) 308-309.
31 Gupta, A., Sakhuja, V., Gupta, K. L. and Chugh, K. S. Intravascular hemolysis and acute renal failure following intermittent rifampin therapy. Int. .J. Lepr. 60 (1992) 185-188.
32 Kakaiya, R. M., Dehertogh. D., Walker, F. J., Cummings, E. and Uzdejczyk, M. Rifampin-induced immune thrombocytopenia; a case report. Vox Sang. 57(1989) 185-187.
33 Ramachandran, A. and Bhatia, V. N. Rifampicin induced shock-a case report. Indian J. Lepr. 62 (1990) 228-229.
34 Brasil. M. T. L. R. F, Opromolla, D. V. A., Marzliak, M. L. and Nogueira. W. Results of a surveillance system for adverse effects in leprosy's WHO/MDT. Int. J. Lepr. 64 (1996) 97-104.
35 Aquinas, S. M., Allan, W. G. L., Horsfall, P. A. L., Jenkins, P. K., Hung-Yan, W., Girling, D., Tall. R. and Foa, W. Adverse reaction to daily and intermittent rifampicin regimens for pulmonary tuberculosis in Hong Kong. Br. Med. J. 1 (1972) 765-771.
36 Riska, N. and Mattson. K. Adverse reactions during rifampicin treatment. Scand. J. Respir. Dis. 53(1972) 87-96.
37 Roth well, D. L. and Richmond, D. E. Hepatorenal failure with self-initiated intermittent rifampicin therapy. Br. Med. J. 2 (1974) 481-482.
38 Mattson, K. and Riska, N. Effect of cortisone on systemic reactions provoked by rifampicin. Scand. J. Respir. Dis. 53 (1972) 97-100.
39 Dukor. P., Schumann, G. and Dietrich, F. M. Immunological studies with rifampicin. Scand. J. Respir. Dis. Suppl. 84 (1973) 73-82.
40 Pujet, J. C, Homberg, J. C. and Decroix, G. Sensitivity to rifampicin: incidence, mechanism and prevention. Br. Med. J. 2 (1974) 415-418.
1. M.D. M.D. Assistant Professor, Division of Dermatology, School of Medicine of Ribeirão Preto, University of Sao Paulo, 14049-900 Ribeirão Preto, SP, Brazil.
2. M.D. Senior Resident, Division of Hematology, School of Medicine of Ribeirão Preto, University of Sao Paulo, 14049-900 Ribeirão Preto, SP, Brazil.
3. M.D., Ph.D. Associate Professor, Division Dermatology, Department of Internal Medicine, School of Medicine of Ribeirão Preto, University of Sao Paulo, 14049-900 Ribeirão Preto, SP, Brazil.