- Volume 65 , Number 3
- Page: 375–8
Minimal inhibitory concentrations of lomefloxacin and minocycline against drug-sensitive and drug-resistant isolates of M. tuberculosis compared on L-J and 7H11 media
ABSTRACT
The in vitro activity of lomefloxacin and minocycline was tested against 46 strains of M. tuberculosis resistant to streptomycin (S), isoniazid (H) and rifampin (R) or SHR and 51 strains sensitive to SHR by the minimal inhibitory concentration (MIC) method on two different media, namely, Lowenstein-Jensen (L-J) and Middlebrook 7H11. The results of the study showed that, irrespective of the medium used, minocycline had little activity against the strains tested and the MIC was >64 µ g/ml. The MIC of lomefloxacin in 7H11 medium ranged f rom 2 to 16 µ g/ml. There were highly significant differences in the MICs of lomefloxacin in L-J compared with 7H11. The results suggest that the activity of lomefloxacin against M. tuberculosis merits further study.To the Editor:
Rifampin derivatives, β-lactam antibiotics with β-lactamase inhibitors, and fluoroquinolones are the newer and highly promising drugs against tuberculosis. Among them, little is known about the activity of lomefloxacin, a new difluoropiperazinyl quinolone (10, 11, 14), and minocycline, a long-acting tetracycline derivative, against Mycobacterium tuberculosis. Minocycline has been studied with respect to its effect on M. leprae only (4, 6-8).
In the present study, we have tested a total of 97 M. tuberculosis strains for their susceptibility to lomefloxacin and minocycline by the minimal inhibitory concentration (MIC) method using both Lowenstein-Jensen (L-J) medium and 7H11 medium to see if the high protein content of L-J medium would have any effect on the MICs. The strains tested included 46 M. tuberculosis strains resistant to S (streptomycin) H (isoniazid) R (rifampin) /HR and 51 susceptible to SHR isolated from patients. Their susceptibility to ciprofloxacin and ofloxacin had been determined previously in our Centre (17).
Lomefloxacin (Torrent Phamaceuticals) and minocycline [Cyanamid of Great Britain Limited (Lederle), kindly provided by Dr. M.D. Guptc, Officer-in-Charge, CJ1L Field Unit, Avadi] at the final concentrations of 16.0, 8.0, 4.0, 2.0, 1.0. 0.5 and 0.25 µ g/ml, and 64, 32. 16, 8, 4, 2 and 1 µ g/ml, respectively, were tested in L-J medium and 7H11 medium containing oleic acid-albumin-dextrose (OADC) enrichment using standard procedures. The inoculated media were incubated at 37ºC with the 7H11 plates kept in 5% CO,, and read at the end of 4 weeks. The lowest concentration of the drug which inhibited growth to <20 colonies compared to at least ++ (numerous discrete colonies) growth on drug-free medium was taken as the minimal inhibitory concentration (MIC).
RESULTS AND DISCUSSION
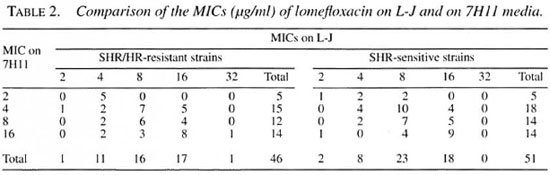
Lomefloxacin. The MIC for the standard M. tuberculosis strain H37Rv was 4 µ g/ml on L-J and 2 µ g/ml on 7H 11. For the other strains, the range of MICs was 2-32 µ g/ml on L-J and 2-16 µ g/ml on 7H11 (Table 1). A highly significant difference between the mean MICs of lomefloxacin on L-J and 7HII was observed, MICs being generally higher on L-J. Of the 46 resistant strains, 16 had the same MICs on L-J and 7H11, 22 had higher MICs on L-J and only 8 had lower MICs on L-J (Table 2). Similarly, of the 51 sensitive strains, 21 had the same MICs on L-J and 7HI1, 23 had higher MICs on L-J while only 7 had lower MICs on L-J. The geometric mean MICs for SHR/HR-resistant and SHR-sensitive strains were 0.84 and 0.82, respectively, on 7HI1, and 0.94 for both resistant and sensitive strains on L-J. The differences in MICs on L-J and 7H11 were highly significant for both resistant and sensitive strains together (p -0.0013), for resistant strains alone (p = 0.0209) and for sensitive strains alone (p = 0.0251). It has been reported earlier that the MICs of quinolones (norfloxacin, perfloxacin, ciprofloxacin and ofloxacin) may not vary much when the agar or broth dilution methods are used (15).
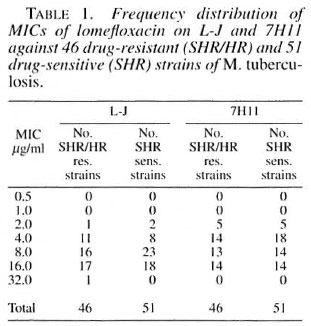
Fluoroquinolones have promising in vitro activity and low toxicity and no crossresistance has been reported between fluoroquinolones and other anti-tuberculosis drugs (12). In earlier studies comparing the activities of different fluoroquinolones, the activity of lomefloxacin has either been less (10) or has compared favorably (14). In the present study, the MICs of lomefloxacin (geometric mean MIC 0.94) were significantly higher than those of ciprofloxacin (geometric mean MIC 0.3) and ofloxacin (geometric mean MIC 0.3) on L-J for the same strains reported in an earlier study from this Centre (17). The MIC ranged from 2-16 µ g/ml for lomefloxacin compared to 1-4 µ g/ml for ciprofloxacin and ofloxacin for the same strains. However, these concentrations of lomefloxacin are probably within the levels achieved in tissues and macrophages because of its pharmacokinetic features, which include a high degree of tissue distribution, a lack of significant metabolism, good oral absorption, long scrum half-life, good tolerance on oral administration, and high tissue and intracellular concentrations (1, 2, 3, 5, 9, 13). Thus, the activity of lomefloxacin against M. tuberculosis merits further study.
Minocycline. The MIC of minocycline was >64 µ g/ml for all of the strains tested both on L-J and 7H11. There was more than 1+ growth (>100 colonies) of all the strains tested even at the concentration of 64 µ g/ml of minocycline, indicating no activity at all of this drug at these concentrations against the M. tuberculosis strains tested. There is to date very meager information on the activity of minocycline against mycobacteria other than M. leprae. In an earlier report, of 5 M. tuberculosis strains tested, 4 were inhibited at 6.5 µ g/ml, and all 5 at 12.5 µ g/ml when Ogawa egg medium was used (16). The serum level is about 2 µ g/ml after a single oral dose of 150 mg of minocycline. Thus, the results of the present study suggest that minocycline may not be useful in the treatment of tuberculosis.
REFERENCES
1. BALDWIN, D. R., WISE, R., ANDREWS, J. M., GILL, M. and HONEYBOURNE, D. Comparative bronchoalveolar concentrations of ciprofloxacin and lomefloxacin following oral administration. Respir. Med. 87(1993)595-601.
2. BELLIVEAU, P., NIGHTINGALE, C. H., QUINTIANI, R., CROWE, H. and GOUSEE, G. Oral ciprofloxacin, ofloxacin, and lomefloxacin as alternatives to intravenous antimicrobial therapy. Conn. Med. 57(1993)539-545.
3. DIAO, Y., Lu, J., Li, L., ZHU, X. D., JI. G. and WANG, E. H. [Pharmacokinetics and relative bioavailability of lomefloxacin, preparations in 10 healthy Chinese volunteers.] Chung Kuo Yao Li Hsueh Pao 14(1993)247-249.
4. FAJARDO, T. T. JR., VILLAHERMOSA, L. G., DELA CRUZ, E. C, ABALOS, R. M., FRANZBLAU, S. G. and WALSH, G. P. Minocycline in lepromatous leprosy. Int. J. Lepr. 63(1995)8-17.
5. FREEMAN, C. D, NICOLAU, D. P., BELLIVEAU, P. P. and NIGHTINGALE, C. H. Lomefloxacin clinical pharmacokinetics. Clin. Pharmacokinet. 25(1993)6-19.
6. GELBER, R. H, FUKUDA, K., BYRD, S., MURRAY, L. P., SIU, P., TSANG, M. and REA, T. H. A clinical trial of minocycline in lepromatous leprosy. Br. Med. J. 304(1992)91-92.
7. GELBER, R. H., MURRAY, L. P., SIU, P., TSANG, M. and REA, T. H. Efficacy of minocycline in single dose and at 100 mg daily for lepromatous leprosy. Int. J. Lepr. 62(1994)568-573.
8. JI, B., JAMET, P., PERANI, E. G., BOBIN, P. and GROSSET, J. H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J. Infect. Dis. 168(199.3)188-190.
9. KAVI, J., STONE, J., ANDREWS, J. M.. ASHBY, J. P. and WISE., R. Tissue penetration and pharmacokinetics of lomefloxacin following multiple doses. Eur. J. Clin. Microbiol. Infect. Dis. 8(1989)168-170.
10. KAWAHARA, S., KAMISAKA, K., TADA, A.. NAKADA, H., MISHIMA, Y., YOSHIMOTO. S., MATSUYAMA, T., KIBATA, M. and NAGARE, J. [ ln vitro activities of newly developed quinolones, fleroxacin, lorne-Moxacin and sparlloxacin against Mycobacterium tuberculosis. ] Kekkaku 66(1991)429-431.
11. MAYER, K. H. and ELLA, J. A. Lomelloxacin, microbiologic assessment and unique properties, Am. J. Med. 92(1992)585-625.
12. PARENTI, F. New experimental drugs for the treatment of tuberculosis. Rev. Infect. Dis. 11 Suppl. 2(1989)S479-S483.
13. PEREA, E. J., GARCIA, I. and PASCUAL, A. Comparative penetration of lomelloxacin and other quinolones into human phagocytes. Am. J. Med. 92(1992)485-515.
14. PIERSIMONI, C, MORBIDUCCI, V.. BORNIGIA, S., DE STO, G. and SCALISE, G. In vitro activity of the new quinolone lomelloxacin against Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 146(1992)1445-1447.
15. TEXIER-MAUGEIN, J., MORMEDE, M., FOURCHE, J. and BEBEAR, C. In vitro activity of new fluoroquinolones against eighty-six isolates of mycobacteria. Eur. J. Clin. Microbiol. 6(1987)583-586.
16. TSUKAMURA, M. In vitro antimycobacterial activity of minocycline. Tubercle 61(1980)37-38.
17. VENKATARAMAN, P., PARAMASIVAN, C. N. and PRABHAKAR, R. In vitro activity of ciprofloxacin and ofloxacin against South Indian isolates of Mycobacterium tuberculosis. Indian J. Tuberc. 41(1994)87-90.
Ph.D. Department of Bacteriology, Tuberculosis Research Center, (Indian, Council of Medical Research) Spur Tank Road, Chetput, Madras 600 031, India
Reprint requests to Dr. Paramasivan atLhe above address or FAX 91-44-8262135.