- Volume 65 , Number 3
- Page: 305–19
Steroid therapy for paralytic deformities in leprosy
ABSTRACT
One-hundred-forty-nine patients with 272 nerve paralyses, with visible deformity and gross disability, were prospectively followed up with steroid therapy. Out of 151 ulnar paralyses, 101 recovered (67%). Out of 52 median nerve paralyses, 45 recovered (86%); out of 69 foot drops, 54 recovered (78%) for an overall improvement of 73%. Serious side effects were few. Hence, steroid therapy should be widely encouraged for the treatment of early nerve damage to prevent permanent deformity/disability, and vigilance in spotting complications of steroid therapy is emphasized.RÉSUMÉ
Cent quarante-neuf malades de la lèpre présentant 272 paralysies nerveuses, avec des déformités visibles et d'importantes incapacités, ont été suivis prospectivement lors de leur traitement par stéroides. Des 151 paralysies cubitales, 101 ont récupéré (67%). Des 52 paralysies du nerf médian, 45 ont récupéré (86%); des 69 pieds tombants, 54 ont récupéré (78%), donnant une amélioration globale de 73%. Les effets secondaires importants ont été peu nombreux. Le traitement par stéroides devrait donc être largement encouragé pour le traitement des lésions nervveuses précoces afin de prévenir les incapacités irréversibles, et on insiste sur la vigilance nécessaire pour détecter les complications du traitement par stéroides.RESUMEN
Cientocuarenta y nueve pacientes con lepra con 272 parálisis de nervios, con visible deformidad y severa incapacidad, fueron tratados con terapia esteroidal y supervisados durante y después del tratamiento. De las 151 parálisis ulnares, 101 se recuperaron (67%). De las 52 parálisis de los nervios medianos, 45 se recuperaron (86%); de 69 pies caídos, 54 se recuperaron (78%). La mejoría global fue del 73%. Los efectos colaterales graves fueron pocos. Con base en estos resultados, recomendamos ampliamente la terapia esteroidal para el tratamiento del daño nervioso temprano con el fin de prevenir las deformaciones y las discapacidades permanentes de la lepra. Se enfati/.a también la necesidad de vigilar las posibles complicaciones de la terapia esteroidal.Leprosy can cause damage to the cranial nerves (especially the zygomatic branch of the facial nerve) and to many peripheral nerve trunks in many patients. This nerve damage leads to visible paralytic deformities (lagophthalmos in facial paralysis, claw hand in ulnar paralysis, ape thumb in median paralysis and foot drop in common peroneal paralysis). The nerve damage can occur whether the patient is on dapsone monotherapy or on multidrug therapy (MDT) and sometimes even after completion of chemotherapy (3, 8, 9). Timely intervention with steroid therapy has been found to be effective in reverting the disabilities to various degrees in a varying proportion of patients (16, 19'). We present here our experience in treating paralytic deformities with long-term steroid therapy over a period of 5 years.
PATIENTS AND METHODS
Patients
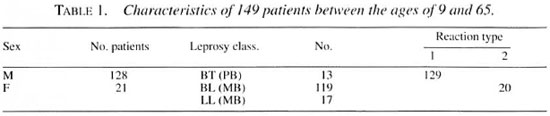
All leprosy patients (both sexes) attending the Sacred Heart Leprosy Centre hospital July 1989-June 1994 with paralysis of the hands and feet of a duration of 6 months or less were included in the study, totaling 156 patients. Seven patients dropped out from follow up for various reasons: 1 for disabilities and cellulitis, 1 for peptic ulcer perforation, and 5 defaulted after 1 month and did not return. The details of the 149 patients available for the study are given in Table 1.
Inclusion criteria
Inclusion criteria included: a) The muscle power of the affected nerves should be less than the Medical Research Council (MRC, London, U.K.) grade 2 (0-2 or less than normal range of movement), b) In case of ulnar nerve paralysis, there should be an obvious mobile claw hand (at least the little finger must show obvious claw deformity and difficulty in grasping function), c) In median nerve paralysis, the ability to oppose the thumb to the pulp of other fingers must be lost at the time of inclusion into the study, d) In common peroneal nerve paralysis, there should be an obvious foot drop with loss of normal heel-toe gait.
Muscle assessment Muscle power was graded as 0-5 based on the MRC scale.
Score. The 0-5 scoring is defined as: 0 = paralysis; 1 = flicker; 2 = limited range of motion, visible movement; 3 = full range of motion, no resistance; 4 = full range of motion, reduced resistance; 5 = normal against resistance.
Ulnar nerve. The following muscles were examined: abductor digiti minimi; 3rd and 4th lumbricales and the first dorsal interosseus.
Median nerve. The abductor pollicis brevis and the opponens pollicis muscles were examined. Common peroneal. The tibialis anterior and the peronei muscles were examined.
Sensory assessment
Only the hands were included for this sensory assessment study. The palms of the hands were assessed in detail using graded nylon monofilaments.
Ulnar nerve. The hypothenar eminence and the little finger base and pulp were assessed.
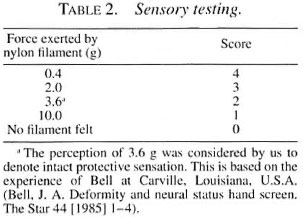
Median nerve. The thenar eminence and the pulp of the thumb, index and long fingers were assessed. Four monofilaments were used. The force of the filaments and the scoring system used in this study are shown in Table 2.
Other investigations
A urinalysis and a hemogram were done. The renal and liver functions, blood sugar, body weight and blood pressure were all examined and recorded initially and re-examined periodically thereafter.
Nerve palpation
The nerves were palpated and thickening, tenderness or pain was recorded.
Treatment
Most of the patients were admitted to the hospital at the initiation of treatment for the first 2-4 weeks.
Prednisolone was the steroid used in our study. In addition, all patients received MDT according to their paucibacillary/multibacillary (PB/MB) status. Prednisolone was given as a single dose every day in the morning and in the dosages shown below:
a) Patient body weight above 40 kg:

b) Patient body weight below 40 kg:
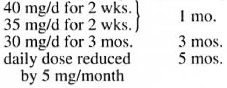
c) Children were treated as follows:

The regimens shown above were possible only in patients suffering from borderline leprosy with a reversal reaction. Patients suffering from an erythema nodosum leprosum (ENL) reaction required longer durations of prednisolone since their reactions kept recurring for longer periods. If there was noticeable improvement in muscle paralysis within the first 3 months of initiating steroid therapy, a tapering off of the steroid dose was started from month 4 onward. If there was neither deterioration nor improvement on reaching the dose of 10 mg/d of prednisolone, then the patient was maintained on 10 mg/d for 3 months, 5 mg/d for another 3 months, and 2.5 mg/d for a final 3 months. All adverse effects during the steroid therapy were carefully watched for and recorded promptly. Active and passive exercises and splinting were done as and when necessary.
Criteria for recovery
Motor recovery at end of steroid therapy. The criteria for motor recovery included: a) There should be no visible deformity; no clawing of lingers, opposition of thumb to fingers must be possible, and the feet must regain normal heel-toe gait while walking [voluntary muscle testing (VMT) should have improved to >MRC grade 3]. b) There should be no need for surgical correction, c) The patient must feel satisfied that he/she has resjained good functionalability of the affected limb for personal care, household work and gainful employment.
Sensory recovery. Only the palms OF the hands were included in this recovery assessment. The area OF the palm corresponding to the particular nerve supply should have regained the perception of 3.6 g nylon monofilament as follows: a) ulnar nerve- in the hypothenar area and little finger pulp; b) median nerve-in the thenar area and the pulp of the thumb, index and long fingers. Improvement was noticed after 3 months to 1 year of initiating steroid therapy.
The study OF sensory recovery over the sole OF the foot was not taken up in this study for the following reasons: a) At the time OF the initiation of the study, the normal sensory threshold for the sole of the foot had not been standardized (van Brakel reported in his 1994 Ph.D. thesis that a 2-g filament force was the normal plantar threshold in healthy Nepalese volunteers), b) Birkc and Sims (5) and Hammond and Klenerman (10) have claimed a 10-g filament force as the protective sensory threshold for the sole OF the foot, and suggested that the inability to perceive 10 g over the sole may indicate the risk of developing plantar ulcers.
However, we carried out a pilot study in the years 1988 and 1989 on some healthy volunteers in South India and found that because of walking barefoot, which is so very common in our South Indian population, many had such thick callosity over their soles that they could not perceive a 10-g filament force, especially over the heels. Currie had also reported similar findings from ALERT in Ethiopia at the XIV International Leprosy Congress in Orlando, Florida, U.S.A., in 1993.
RESULTS
Of the 149 patients available for follow up after completion of steroid therapy, 30 patients (20%) could be followed up for 6 months and 119 patients (80%) could be followed up for 1 to 5 years. The results are given in Table 3.
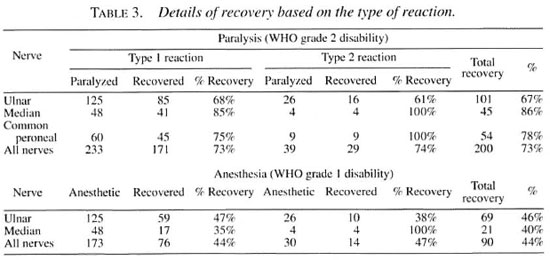
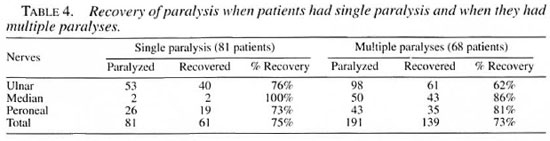
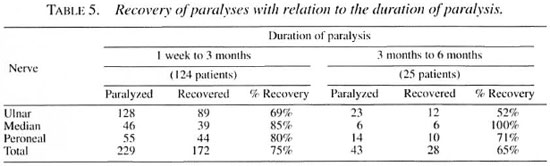
The overall recovery rate was similar in type 1 and type 2 reactions (Table 3), and also similar whether the patient had a single paralysis or multiple paralyses (Table 4) but in the patients with multiple paralyses, although the duration of paralysis was the same in each individual patient, some nerves recovered and others did not, which is strange and frustrating (e.g., when a patient with bilateral ulnar paralysis which occurred simultaneously was treated, one hand recovered and the other hand did not). Similarly, a patient with ulnar median paralysis in one hand showed recovery of the median nerve alone, leaving behind the ulnar paralysis. Of course, some patients with bilateral ulnar, median and peroneal paralyses gained complete recovery of all of these nerves. Recovery was definitely better when the duration of paralysis was <3 months (Table 5). Two patients with ulnar paralysis who had persistent clawing of lingers at the time of cessation of steroid therapy presented after 1 year with remarkable improvement with no visible deformity.
Initial improvement and recurrence of paralysis
A total of 12 nerves (8 ulnar, 2 median and 2 common peroneal) showed very good improvement in the beginning but the paralysis recurred at the time of tapering the prednisolone dosage and did not improve further in spite of increasing the dosage of prednisolone. We could not explain the reason for this phenomenon. These nerves were included under the "not improved" category.
Paralysis after stopping steroid therapy
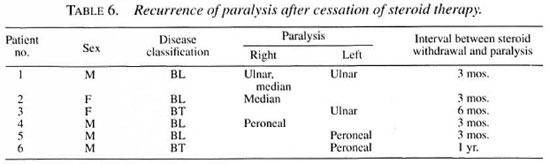
Six patients developed a recurrence of paralysis after stopping the steroid therapy. All of the patients were borderline patients with type 1 reaction [2 borderline tuberculoid (BT) and 4 borderline lepromatous (BL) patients], resulting in 8 paralyses (3 foot drop, 3 ulnar paralysis with clawing of fingers, 2 median paralysis) (Table 6). Patients 4, 5 and 6 with foot drop received another course of steroid therapy. Foot drop recovered well in two patients (4 and 6) and did not improve in the other patient (no. 5). For the other 3 patients (3 ulnar paralysis and 2 median paralysis) surgical correction has been advised. Patient 3 (a 20-year-old woman) was apprehensive about the side effects of the steroids and opted voluntarily to have surgical correction. Patients 1 and 2 were advised to have surgical correction because they had already received long-term steroid therapy (>1 year) and, hence, we were apprehensive of the steroid side effects and did not want to restart long-term steroid therapy.
Adverse effects of steroid therapy
The complications encountered while the patients received steroid therapy are discussed briefly below and in Table 7.
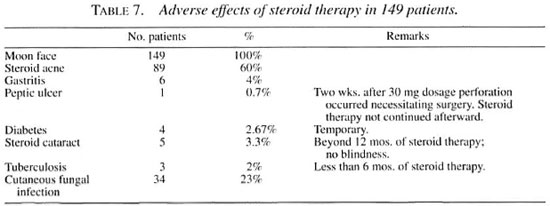
Moon face. "Moon face" was a universal phenomenon, ranging from a mild degree to complete rounding up of the face. It was embarrassing and distressing as well as of great concern to all patients and their relatives. Reassurance was given. Mooning started 2 weeks after initiation of the steroid therapy and always subsided after stopping the steroids.
Steroid acne. Steroid acne was the next common side effect noticed. Acneiform eruptions occurred 1 month after initiation of the steroid therapy. They were distributed over the face, chest, arms and scapulae. Patients felt distressed due to cosmetic implication. The eruptions subsided after steroid withdrawal.
Cutaneous fungal infection. Cutaneous fungal infection was the next common phenomenon observed. Plaques of fungus lesions occurred mainly over the thighs, abdomen, chest and intertriginous areas, such as the axillae and groin. The infection occurred with 3 months of taking prednisolone, but was controlled well with topical clotrimazole.
Gastritis. Gastritis occurred occasionally at any stage of the steroid therapy, and was neither related to the dosage nor to the duration of steroid therapy. It was relieved by antacids and ranitidine given orally and, therefore, we could continue the steroid therapy without interruption.
Peptic ulcer. A 35-year-old woman developed severe abdominal pain 2 weeks after taking prednisolone 30 mg daily. She was found to have a perforated peptic ulcer at laparotomy. Steroid therapy was discontinued in this patient.
Diabetes. Diabetes occurred within 12 months of steroid therapy, with postprandial blood glucose levels ranging between 230 mg/100 ml and 630 mg/100 ml. Diabetes was easily controlled with oral glibenclamide. Diabetes was always temporary, and blood sugar levels returned to normal when the dose of prednisolone was reduced to <30 mg daily.
Cataract. Cataract occurred among patients who received more than 12 months of prednisolone therapy. There was no blindness but the patients suffered from "glare disabilities," disturbing their vision when they were outdoors in bright sunlight. The visual difficulty was described as "foggy," "cloudy," and "smoky" and vision dramatically improved after dusk. The clarity of their vision also improved if they wore sunglasses during bright daylight. Vision improved spontaneously in one patient 6 months after the cessation of the steroid therapy.
Tuberculosis. Three patients developed pulmonary tuberculosis within 6 months of prednisolone therapy. They were managed well with antituberculosis drugs without stopping the steroid therapy. All three patients (1 BT, 2 BL) had a type 1 reaction.
DISCUSSION
The restoration of nerve function when treated with prednisolone in patients suffering from leprosy reactions has been known for the past 25 years. Clinical and electrophysiological improvement by steroids was demonstrated in ENL patients by Sheskin, et al. and Magora, et al. in 1969 and 1970, respectively (16, 25). Naafs and Dagne and Naafs, et al. demonstrated significant improvement in the neurological status of borderline leprosy patients suffering from reversal reaction in 1976 and 1977, respectively (17, 18). Again, in 1979 Naafs, et al. highlighted the significantly better neurological recovery by long-term steroid therapy when compared to short-term steroid therapy (19). This has been repeatedly confirmed by Srinivasan, et al. in India (27), Touw-Langendijk, et al. in Ethiopia (28), and Kiran, et al. in India (13). [Some of the studies reporting poor outcome of steroid therapy could be due to an inadequate dosage or duration of prednisolone (15, 23).]
We deliberately concentrated on patients with visible deformity and gross disability so that what is possible as the best outcome in this category of patients could be discovered. It was indeed gratifying to see such a high proportion of good recovery from the nerve damage (ulnar claw recovered 67%, thumb paralysis recovered 86%, foot drop recovered 78%). This recovery has greatly reduced the need for these patients to undergo surgical intervention. We feel that the number of nerves studied in this series is quite large and, hence, the significant favorable outcome should encourage many other leprosy projects to boldly introduce steroid therapy with a sufficiently large dose/duration of prednisolone. However, the-small proportion of the nerves showing sensory recovery is both surprising and disappointing (ulnar nerve 46%; median nerve 42%). Further analysis reveals that while 67% of the ulnar nerves improved from a WHO grade 2 disability (101 out of a total of 151), only 68% of these improved nerves also regained protective sensation (69 out of 101), leaving 32 of these improved hands with a WHO grade 1 disability. Similarly, 86% of the paralyzed thumbs regained good muscle power and function (45 out of 52), but only 49% of these nerves regained protective sensation to the median area of these hands (21 out of 45), leaving 24 hands with a WHO grade 1 disability. In other words, when the total number of ulnar and median nerves included for treatment are considered, at the end of the steroid treatment the majority of the nerves persisted with a WHO grade 1 disability (56%, 113 out of 203), which is of great concern to us.
The adverse effects, although few, indicate the need for great vigilance on the part of the physician and other team members treating these patients. We strongly recommend the installation of the necessary skills and laboratory facilities so that problems can be spotted early for prompt remedy. Since the World Health Organization (WHO) published in 1993 a practical guide for the prevention of leprosy deformities with the recommendation to treat all early disabilities (<1 year duration) with adequate steroids, more physicians should enthusiastically start treating patients with steroids to prevent permanent disability/deformity (26). In our present study, we included only paralysis of 6 months or less. Now we plan to include patients with paralysis of up to 1 year to find out the possible benefit of steroid therapy in these patients.
Our dosage and duration of prednisolone more or less resemble the experience of Jacobson (12), working in a referral center in the "First World," and that of Pfaltzgraff (22). Most BT patients require 4-9 months of steroid treatment, BB patients 6-12 months, and BL patients 6-24 months (23).
Muscle assessment by VMT, grading the strength 0-5, and sensory testing using graded nylon monofilaments were found to be useful tools for monitoring nerve function and treatment needs to recognize improvement/deterioration so that the optimum dosage/duration of prednisolone can be determined for each patient individually (4.6,7.9. 17, 19 and Bell J. A. Semmes-Weistein monofilament testing for determining cutaneous lisht touch/deep sensation. The Star 44 [1984] 8-11. Bell, J. A. Deformity and neural status hand screen. The Star 44 [1985] 1-4. Brandsma, J. W. Nerve function testing and evaluation-Part I. The Star 43 [1984] 2-3. Brandsma, J. W. Nerve function testing and evaluation-Part II. The Star 44 [1984]8-10).
CONCLUSIONS
Treatment of paralytic deformities with careful follow up over the past 5 years has enabled us to draw some conclusions.
1. Steroid therapy of paralytic deformities (< 6 months' duration) is very effective in correcting most of the deformities and in restoring function to the affected limbs.
2. We could not discover a standard regimen which could be effective in all patients. Each patient had to be treated individually, tailoring the dosage/duration of the steroid depending upon the response of each patient.
3. Voluntary muscle testing and sensory testing (using graded nylon monofilaments) are excellent parameters for monitoring patients and for providing optimum treatment and, hence, very practical for making reliable, rapid and repeated assessments.
4. Serious side effects of corticosteroids, although few, do occur and, therefore, utmost vigilance is essential to spot complications as and when they occur so that remedial steps can be taken promptly. We feel that suitable laboratory support and extra training regarding the recognition of steroid complications are necessary (11).
5. There appears to be no short cut for the prevention of disability (POD) in leprosy. The chemotherapy of leprosy (MDT) and POD efforts are inseparable for anyone undertaking leprosy control because reactions and nerve damage do occur during treatment and after (whether treatment is MDT or any other form of therapy) (2, 8, 9, 20, 29). This calls for "proper" allocation of resources, always keeping in mind the needs of the patient. It involves providing technical knowledge, imparting suitable skill in POD, laboratory back up, suitable medicines for treating early disability, tackling treatment- related complications, etc. The arbitrary allocation of resources leads to improper planning and results in suboptimal treatment or sometimes total neglect of POD aspects. So, in most of the leprosy projects (both governmental and nongovernmental) the ultimate outcome is "Leprosy cured but nerves died," hence permanent deformity/ disability. (This is somewhat similar to the saying, "Operation successful but patient died.") Hence, the "patient" must be considered as important instead of trying to treat the "bacteria" or the "disease" or the "disability" in isolation.
6. Only one case required surgical decompression (of an ulnar nerve) because she developed perforation of the stomach within a short period of beginning low-dose steroid therapy (30 mg daily for 2 weeks). Hence, the need for surgical intervention in neuritis appears to be extremely rare.
7. Further research is needed to identify the reasons for the failure of a proportion of the nerves to recover, especially the lower incidence of sensory recovery. New approaches to further improve nerve function is warranted since we feel that our experience, along with that of other workers in the past, is just a beginning in the direction of POD.
Acknowledgment. I thank the Superintendent, Sacred Heart Leprosy Centre, for allowing me to conduct this study. Sincere thanks also go to my fellow physicians and to the physiotherapy technicians for their close collaboration and cooperation. My deep gratitude goes to the staff of the Medical Records Department for supplying the records and data as and when required repeatedly. Special thanks go to Mr. S. Seshadri for typing the manuscripts and for secretarial assistance. I am very grateful to D, D. Palande, M.S., and G. Ramu, M.D., Cor their constant encouragement, critical comments and helpful suggestions while conducting this study as well as while preparing this paper.
REFERENCES
1. BECX-BLEUMINK, M. The management of nerve damage in the leprosy control services. Lepr. Rev. 61(1990)1-11.
2. BECX-BLEUMINK, M. Priorities for the future and prospects for leprosy control. (Editorial) Int. J. Lepr. 61(1993)82-101.
3. BECX-BLEUMINK, M. and BERHE, D. Occurrence of reactions; their diagnosis and management in leprosy patients treated with multidrug therapy, experience in the Leprosy Control Programme of the All Africa Leprosy and Rehabilitation Training Centre (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
4. BELL, J. A. The repeatability of testing with Semmes-Weinstein monofilaments. J. Hand Surg. 12A(1987)155-161.
5. BIRKE, J. A. and SIMS, D. S. Plantar sensory threshold in the ulcerative foot. Lepr. Rev. 57(1986)261-267.
6. BRANDSMA, J. W. Basic nerve function assessments in leprosy patients. Lepr. Rev. 52(1981)161-170.
7. G OODWIN, C. S. The use of voluntary muscle testing in leprosy neuritis. Lepr. Rev. 39(1968)209-216.
8. GROENEN, G., JANSSKNS, L., KAYEMBE, T, NOLLET, E., COUSSENS, L. and PATTYN, S. R. Prospective study on the relationship between intensive bactericidal therapy and leprosy reactions. Int. J. Lepr. 54(1986)236-244.
9. HAMILTON, J. Deformity prevention in the field; a systematic approach. Lepr. Rev. 54(1983)229-237.
10. HAMMOND, C. J. and KLENERMAN, P. Protective sensation in the foot in leprosy. Lepr. Rev. 59(1988)347-354.
11. HAYNES, R. C, JR. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs: inhibitors of the synthesis and actions of adrenocortical hormones. In: Goodman and Oilman's Tlie Pharmacological Basis of Therapeutics. 8th edn. Gilman, A. G., Rail, T. W., Nies, A. S. and Taylor, P., eds. New York: Pergamon Press, 1990, pp. 1431-1462.
12. JACOBSON, R. R. Treatment. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, p. 198.
13. KIKAN, K. U., STANLEY, J. N. A. and PEARSON, J. M. H. The outpatient treatment of nerve damage in patients with borderline leprosy using a semi-standardised steroid regimen. Lepr. Rev. 56(1985)127-134.
14. LEWIS, S. Reproducibility of sensory testing and voluntary muscle testing in evaluating the treatment of acute neuritis in leprosy patients. Lepr. Rev. 54(1983)23-30.
15. LOCKWOOD, D. N. J., VINAYAKUMAK, S., STANLEY, J. N. A., MCADAM, K. P. W. J. and COLSTON, M. J. Clinical features and outcome of reversal (type I) reactions in Hyderabad, India. Int. J. Lepr. 61(1993)8-15.
16. MAGORA, A., SHESKIN, J., SAGHKR, E. and GONHN, B. The condition of the peripheral nerve in leprosy under various forms of treatment. Int. J. Lepr. 38(1970)149-163.
17. NAAFS, B. and DAGNE, T. Sensory testing; a sensitive method in the follow-up of nerve involvement. Int. J. Lepr. 45(1977)364-368.
18. NAAFS, B., PEARSON, J. M. H. and BAAR, A. J. M. A follow-up study of nerve lesions in leprosy during and after reactions using motor nerve conduction velocity. Int. J. Lepr. 44(1976)188-197.
19. NAAFS, B., PEARSON, J. M. H. and WHEATE, H. W. Reversal reactions: the prevention of nerve damage; comparison of short- and long-term steroid treatment. Int. J. Lepr. 47(1979)7-12.
20. NAAFS, B. and WHEATE:, H. W. The time interval between the start of antileprosy treatment and the development of reactions in borderline patients. Lepr. Rev. 48(1978)153-157.
21. PEARSON, J. M . H. The evaluation of nerve damage in leprosy. Lepr. Rev. 53(1982)119-130.
22. PFALTZGRAFF, R. E. Therapy of leprosy. Health Coop. Papers 1(1981)121-122 .
23. PFALTZGRAEE, R. E. The management of reactions in leprosy. Int. J. Lepr. 57(1989)103-109.
24. ROSE, P. and WATERS, M. F. R. Reversal reactions in leprosy and their management. Lepr. Rev. 62(1991)113-121.
25. SHESKIN, J., M AGORA. A. and SAGHER, F. Motor conduction velocity studies in patients with leprosy reactions treated with thalidomide and other drugs. Int. J. Lepr. 37(1969)351-364.
26. SRINIVASAN, H. Prevention of disabilities in leprosy-a practical guide. Geneva: World Health Organization, 1993.
27. SRINIVASAN, H., RAO, K. S. and SHANMUGAM, N. Steroid therapy in recent "quiet nerve paralysis" in leprosy-report of a study of twenty-five patients. Lepr. India 54(1982)412-419.
28. TOUW-LANGENDIJK, E. M. J., BRANDSMA, J. W. and ANDERSEN, J. G. Treatment of ulnar and median nerve function loss in borderline leprosy. Lepr. Rev. 55(1984)41-46.
29. WATSON, J. M. Preventing disability in leprosy patients. London: The Leprosy Mission International, 1986.
D. S. T. Sugumaran, M.B.B.S., D.P.M., Department of Medicine, Sacred Heart Leprosy Centre, Sakkottai 612 401, Kumbakonam R.S., Thanjavur District, Tamil Nadu, India.
Present address: Schieffetin Leprosy Research & Training Center, SLR Sanatorium P.O., N.A.A. District 632 106, South India.
Received for publication on 24 October 1996.
Accepted for publication in revised form on 25 March 1997.