- Volume 65 , Number 3
- Page: 345–51
Studies on therapeutic activity of benzoxazinorifamycin KRM-1648 in combination with other antimicrobial agents and biological response modifiers interferon-γ and granulocyte-macrophage colony-stimulating factor against M. leprae infection in athymic nude mice
ABSTRACT
In the present study, we evaluated the in vivo anti -Mycobacteriuni leprae activities OF KRM-1648 (KRM) given at long intervals in combination with ofloxacin (OFLX), clofazimine (CFZ), and dapsone (DDS). We also examined the combined effects of two biological response modifiers (BRMs), gamma interferon (IFN-γ) and granulocytemacrophage colony-stimulating factor (GM-CSF), on the therapeutic efficacy of KRM. KRM exhibited potent therapeutic efficacy against M. leprae infection in mice even when given at 4-week intervals. KRM displayed increased efficacy in combination with OFLX, CFZ, and DDS (given three or six times per week) when given to mice in the multidrug combination KRM+OFLX+ CFZ+DDS. The therapeutic efficacy of KRM given at 4-week intervals was increased by combined use with IFN-γ but not by GM-CSF. Adoptive transfer of M. leprae antigen-primed lymphocytes of euthymic mice to recipient athymic nude mice with progressive M. leprae infection markedly enhanced host resistance.RÉSUMÉ
Dans l'étude présente, nous avons évalué les activités in vivo vis-à-vis de Mycobacterium leprae de KRM-1648 (KRM) donné à de longs intervalles en combinaison avec l'ofloxacine (OFLX), la clofazimine (CFZ) et la dapsone (DDS). Nous avons également examiné les effets combinés de deux modificateurs de réponse biologique (MRB), l'interferon gamma (IFN-γ) et le facteur de stimulation des colonies de granulocytes-macrophages (FSC-GM), sur l'efficacité thérapeutique du KRM. Le KRM a montré une efficacité thérapeutique puissante vis-à-vis de l'infection à M. leprae che/ la souris, même quand il est donné à des intervalles de quatre semaines. Le KRM a montré une efficacité accrue en combinaison avec OFLX, CFX et DDS (donnés trois ou six fois par semaine) quand administré à la souris en combinaison polychimiothérapeutique KRM+OFLX+CFZ+DDS. L'efficacité thérapeutique du KRM donné à des intervalles de quatre semaines était augmentée par la combinaison avec IFN-γ, mais pas par FSC-GM. Le transfert adoptif de lymphocytes de souris euthymiques activés par des antigènes de M. leprae à des souris nues athymiques présentant une infection progressive par M. leprae ont stimlulé la résistance de l'hôte de manière marquée.RESUMEN
En el presente estudio evaluamos la actividad anti- Mycobacterium leprae de la droga KRM-1648 (KRM) administrada en combinación eon ofloxacina (OFLX), clofazimina (CFZ) y dapsona (DDS). a intervalos largos de tiempo. También exeminamos los efectos combinados de dos modificadores biológicos de la respuesta inmune (interferon gamma. IFNγ, y factor estimulante de colonias tic granulocitos y monocitos, GM-CSF) sobre la eficacia terapéutica de KRM. La droga (KRM) exhibió un potente electo terapéutico contra la infección por M. leprae en el ratón, aun administrado a intervalos de 4 semanas. La eficiencia de KRM fue todavía mayor cuando se administró, 3 ó 6 veces por semana, en la combinación KRM+OFLX+ CFZ+DDS. I.a eficacia terapéutica de la KRM administrada a intervalos de 4 semanas, también se incrementó cuando se usó en combinación con IFNγ pero no cuando se usó junto con el GMCSF. La transferencia adoptiva de linfocitos de ratones eutímicos estimulados con antígenos de M. leprae a ratones desnudos atímicos infectados con M. leprae, aumentó marcadamente la resistencia del huésped.Long-term chemotherapy is needed to achieve appreciable results in the control of multibacillary infections with use of multidrug regimen including rifampin, dapsone (DDS), and clofazimine (CFZ) (9, 28). The development of short-term chemotherapy regimens for leprosy patients using new drugs with highly potentiated antileprosy activity is desired in order to simplify administration of therapy by the general health services. Previously, we found that the new benzoxazinorifamycin derivative, KRM-1648 (KRM), displayed excellent in vivo therapeutic efficacy against Mycobacterium leprae infection induced in athymic nude mice (27). In addition, KRM exhibited increased therapeutic efficacy in combination with DDS and CFZ when these drugs were administered to mice once daily for 50 days (20), or in the following protocol for 90 days from day 91 to day 180: KRM, once weekly; DDS, daily, six times per week; CFZ, twice per week (19).
In this study, we evaluated the in vivo anti-M. leprae activities of KRM given at long intervals (once monthly) in combination with ofloxacin (OFLX), CFZ, and DDS, which were given to mice using a different protocol. We also studied the combined effects of two biological response modifiers (BRMs), interferon-gamma (IFN-γ)(16) and granulocyte-macrophage colonystimulating factor (GM-CSF) (6), on the therapeutic efficacy of KRM. We found that IFN-γ increased the therapeutic efficacy of KRM but that GM-CSF antagonized the effect of KRM. Given these findings, we carried out further studies on the modes of participation of T lymphocytes in host resistance to M. leprae infection. We had previously found that the anti-M. leprae resistance of BALB/c athymic nude mice was markedly increased when they underwent adoptive transfer of whole and anti-CD4 monoclonal antibody (mAb)- or anti-CD8 mAb-treated lymphocytes from euthymic mice, 1 to 3 weeks prior to infection (12). In that study, all three lymphocyte preparations augmented host resistance to a comparable extent. This suggests that both CD4+ and CD8+ T cells are required for expression of host resistance to M. leprae infection.
In the present study, we performed lymphocyte transfer for recipient mice in the progressive stage of M. leprae infection, and found that adoptive transfer of lymphocyte preparations using such a protocol effectively decreased bacterial growth.
MATERIALS AND METHODS
Mice. Female, 6-week-old, BALB/c athymic nude (nu/nu) mice and 8- to 10-week-old, BALB/c eutliyniic (+/+) mice were purchased from Japan Clea Co., Tokyo,Japan.
Organisms. M. leprae Thai-53 strain were obtained from Dr. M. Matsuoka, National Institute for Leprosy Research, Tokyo,Japan.
Special agents. KRM, OFLX, and CFZ were kind gifts from Kaneka Corporation, Hyogo, Japan; Daiichi Pharmaceutical Co., Tokyo, Japan, and Ciba-Geigy Co., Tokyo, Japan, respectively. DDS was obtained from Wako Pure Chemical Industries, Osaka, Japan. Monoclonal rat anti-mouse L3T4 (CD4) and mouse anti-Lyt-2.2 (CD8) antibodies (Abs) were purchased from Caltac Laboratories Inc., South San Francisco, California, U.S.A. and Cedarlane Laboratories, Ontario, Canada, respectively. Fluorescein isothiocyanate (FITC)-conjugated rat antimouse CD4 and FITC-conjugated rat antimouse CD8a mono- clonal antibodies (mAbs) and phycoerythrin (PE)-conjugated hamster anti-mouse CD3-E mAb were purchased from Pharmingen Co., San Diego, California, U.S.A. Recombinant mouse IFN-γ was supplied by Daiichi and recombinant mouse GM-CSF was obtained from Upstate Biotechnology, Inc., Lake Placid, New-York, U.S.A. One unit of IFN-γ was defined as the amount required to protect 50% of the indicator cell population (L929 cells) from viral (vesicular stomatitis virus) destruction. One unit of GM-CSF was defined as the amount required to induce half-maximal stimulation of granulocyte and macrophage colony formation from murine bone marrow cells.
Experimental infection. M. leprae were harvested from infected foot pads (FPs) of BALB/c nude mice, and bacterial suspensions were prepared as follows (27): The infected FPs were homogenized in Hanks' balanced salt solution (HBSS) containing 5% fetal bovine serum (FBS) (Bio Whittaker Co., Walkersville, Maryland, U.S.A.) and were centrifuged at 200 x g x 5 min. The upper layer was saved, and the bacilli were collected by recentrifugation at 1500 x g x 15 min. The bacterial suspension was again centrifuged at 200 x g x 5 min, and the upper layer was used as an inoculum for experimental infection. Six-week-old, BALB/c nude mice (15 ± 1 g body weight) were infected subcutaneously with 106 of M. leprae into the left hind FPs. One year later, mice were killed and the number of acid-fast bacilli (AFB) in the FP was counted using the method of Shepard (23).
Chemotherapy. KRM, DDS, OFLX, and CFZ finely emulsified or dissolved in 0.1 ml of 2.5% gum arabic-0.1 % Tween 80 solution were given to infected mice by gavage from day 91 to day 180 using the following protocols: KRM, once every 4 weeks; OFLX and CFZ, three times per week; DDS, daily six times per week. IFNyand GM-CSF dissolved in 0.1 ml of 0.1% BSA solution were injected intramuscularly once weekly into infected mice from day 91 to day 180. Although each drug was given to mice at per mouse doses, the variances of body weights of mice of the test regimens were relatively small: 27.1 ± 0.4 g (mean ± S.E.M.; N = 40) at 1 year after infection.
Priming of mice with M. leprae antigens. Gamma-ray-irradiated armadillo liver (3825) naturally infected with M. leprae was obtained from G. P. Walsh, Armed Forces Institute of Pathology, Washington, D.C., U.S.A. From this M. leprrae -infected liver, M. leprae were purified following the "IMMLEP protocol 1/79," a Percoll gradient centrifugation technique, as previously reported (18). BALB/c euthymic mice were injected intravenously with 1 x 107 of the purified M. leprae. Mice were sacrificed to prepare M. leprae antigen-primed lymphocytes for adoptive transfer 4 weeks after the antigen priming.
Adoptive lymphocyte transfer. Adoptive transfer of whole and anti-CD4 or anti-CD8 mAb-treated lymphocytes to the recipient athymic nude mice was performed as previously described (12). Briefly, spleen cells (SPC) from BALB/c euthymic mice primed or unprimed with M. leprae were suspended in RPMI-1640 medium supplemented with 5% FBS (M. A. Bioproducts, Walkersville, Maryland, U.S.A.), and applied to a Sephadex G-10 column to remove adherent cells. The resultant lymphocyte fraction was then treated with anti-L3T4 (CD4) mAb (200 µ g/ml), anti-Lyt-2.2 (CD8) mAb (200 µ g/ml), or with medium alone at 0ºC for 1 hr. After washing, the cells (7 x 107) suspended in 0.2 ml of RPMI-1640 medium were transferred intravenously to BALB/c athymic nude mice.
In a separate experiment, each cell preparation used for adoptive transfer was further treated with rabbit complement (Cedarlane) at 1:2 dilution at 37ºC for 1 hr, and then analyzed for the content of CD4+ and CD8+ T cells in the resulting cell suspensions after staining with either FITC-conjugated anti-CD4 or anti-CD8 mAb in combination with PE-conjugatcd anti-CD3 mAb, using FAC-STAR (Becton Dickinson, Mountain View, California, U.S.A.). The anti-L3T4 mAbtreated and anti-Lyt-2.2 mAb-treated lymphocyte fractions were found to be almost free from remaining CD4+ T cells (only 1.7%; markedly decreased from the value of original unfractionated lymphocytes, 25.3%) and CD8+ T cells (only 0.8%; markedly decreased from the value of unfractionated lymphocytes, 19.9%), respectively, thereby indicating a sufficient level of anti-CD4 Ab- and antï-CD8 Ab-sensitization of the lymphocyte fractions used in our adoptive transfer experiments. Therefore, it is thought that adoptive transfer of the present anti-L3T4 mAb-treated and anti-Lyt-2.2 mAb-treated lymphocyte fractions might result in the reconstitution of T lymphocytes essentially lacking in CD4+ and CD8+ T cells, respectively, in the recipient mice, due to in vivo lytic action of host blood complement against the Ab-sensitized cells.
Statistical analysis. Statistical multiple comparisons of the bacterial loads in the infected FPs of mice given each of the regimens was performed by Bonferroni's multiple / test according to Godfrey (7).
RESULTS
Table 1 shows the therapeutic efficacies of KRM (given once every 4 weeks), OFLX and CFZ (given three times per week), and DDS (given daily six times per week) alone or in multidrug combinations of KRM with the other drugs against M. leprae infection in mice. KRM, OFLX, CFZ, and DDS were given at doses of 0.2, 0.25, 0.05, and 0.05 mg/mouse (equivalent to clinical dosages), respectively. KRM and CFZ exhibited significant antileprosy activity, causing a 4.12 and 4.18 log-unit decrease, respectively, in the number of AFB per FP compared to the value for the control mice (8.77 ± 0.19 log units). DDS had a weaker but still significant therapeutic efficacy, causing a 1.28 log-unit decrease. In contrast, OFLX exhibited no significant efficacy against M. leprae infection. Among multidrug combinations tested, neither KRM+OFLX+CFZ nor KRM+OFLX+ DDS exhibited significant combined effects as compared to the effect of CFZ or KRM or DDS monotherapy, causing a 4.35 and 4.46 log-unit decrease, respectively. The four-drug combination of KRM+OFLX+ CFZ+DDS did exhibit a combined effect, causing a 5.10 log-unit decrease. This multidrug combination caused significantly reduced bacterial loads as compared to those of mice given KRM, OFLX, CFZ, or DDS alone (p < 0.05 or p < 0.01; Bonferroni's multiple t test), although there were no significant differences for KRM4-OFLX+ CFZ+ DDS vs KRM+OFLX+CFZor KRM+ OFLX+ CFZ+ DDS vs KRM+OFLX+ DDS. In this experiment, a significant decrease in FP swelling during the course of M leprae infection was observed in mice given the test drugs. However, administration of KRM with the other drugs did not reduce FP thickness.
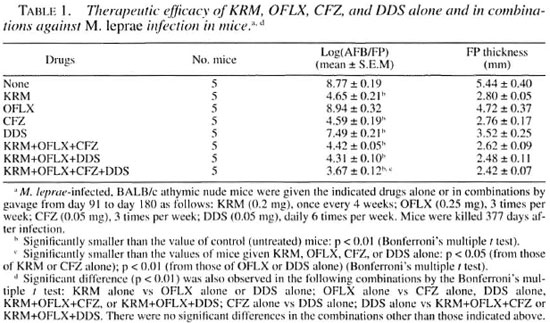
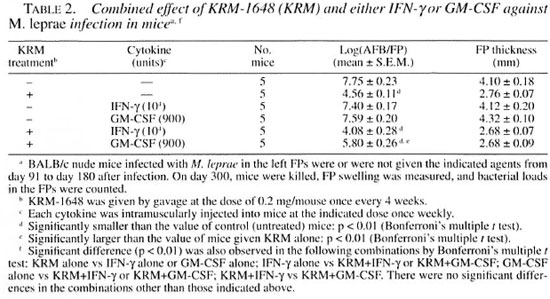
Table 2 shows the therapeutic efficacies of KRM and two pro-inflammatory cytokines (IFN-γ and GM-CSF) alone or in combinations of KRM with the cytokines against M. leprae infection in mice. KRM exhibited a potent therapeutic efficacy, causing a 3.19 log-unit decrease, even when administered at intervals as long as 4 weeks. The two cytokines given at 1-week intervals slightly reduced the growth of M. leprae compared with that in control mice, i.e., a 0.35 and 0.16 log-unit decrease in mice given IFN-γ and GM-CSF, respectively. In this case, the observed reduction in the bacterial loads was not statistically significant in Bonferroni's multiple t test. When KRM was given to mice in combination with IFN-γ, a weak combined effect was obtained with further reduction in the number of AFB, i.e., a 3.67 log-unit decrease. In this case, the difference in the bacterial loads between KRM alone and KRM+IFN-γ was not significant in Bonferroni's multiple t test. In contrast, when KRM was given in combination with GM-CSF, the therapeutic efficacy of KRM was antagonized by GM-CSF, with only a 1.95 log-unit decrease in the number of AFB obtained. In this case, the bacterial loads in mice given KRM+GM-CSF were significantly larger than those in mice given KRM alone (p < 0.01; Bonferroni's multiple t test). Thus, the in vivo anti- M. leprae activity of KRM appears to be potentiated by combination with immunotherapy using IFN-γ but not that with GM-CSF.
We then studied the roles of CD4+ and CD8+ T cells in expression of host resistance to M. leprae infection as follows: Splenic lymphocytes were obtained from BALB/c euthymic mice which had been primed with M. leprae antigen by intravenously injecting gamma-irradiated organisms 4 weeks before cell harvest. Then, whole and anti-CD4 or anti-CD8 mAbtreated lymphocytes were adoptively transferred to BALB/c athymic mice (four mice per regimen) in the stage of progressive M. leprae infection, i.e., 14 weeks after the bacterial challenge. All of the lymphocyte preparations markedly reduced the growth of M. leprae in recipient mice (data on the adoptive transfer experiments not shown). In all cases, the numbers of organisms recovered from the infected FPs 368 days after infection were lower than or just at the limit of detection (<3.25 to 3.66 log-units). In this experiment, whole lymphocytes from M. leprae -primed donor mice inhibited the growth of M. leprae at the sites of infection, as previously reported by Shannon, et al . (22). M. leprae antigen-primed lymphocytes potentiated host resistance to the same extent as did unprimed lymphocytes in terms of decreased bacterial loads in infected FPs as follows: unprimed cells, 3.31 logunits/FP; primed cells, 3.34 log-units/FP.
DISCUSSION
In this study, KRM exhibited potent therapeutic efficacy against M. leprae infection in mice even when given at long intervals (once per month). KRM displayed increased efficacy in combination with OFLX, CFZ, and DDS, which were given three or six times per week at doses equivalent to their clinical dosages. Based on the present and previous findings (19, 20), we can conclude that KRM displayed increased efficacy when used in the following combinations: KRM+CFZ, KRM+DDS, KRM+ CFZ+DDS, and KRM+OFLX+CFZ+DDS. However, no significant combined effect was observed for other combinations including KRM+CFZ (19), KRM+OFLX+ CFZ, and KRM+OFLX+DDS (Table 1). It is unclear why neither KRM+OFLX+CFZ nor KRM+OFLX+DDS exhibited combined efficacy while both KRM+CFZ and KRM+DDS did. A possible explanation for this finding is that OFLX might inhibit the potentiation by either CFZ or DDS of the therapeutic efficacy of KRM by an unknown mechanism. In addition, this competitive effect of OFLX would disappear when it was added to the combination KRM+CFZ+DDS. Therefore, it is important to verify such possibilities. Furthermore, our previous study (26) indicated that a much larger dose of OFLX was needed (3.0 mg/mouse) than that in our present study (0.25 mg/mouse; ca. 10 mg/kg) to cause a significant bacterial response in the treated mice. Indeed, pharmacokinetic studies showed that the serum level of OFLX after oral administration at 10 mg/kg in mice was about five times lower than that achieved in humans given the same dosage of OFLX on the basis of body weight: that is, Cmax in mice and humans were reported to be 1.21 and 6.64 µ g/ml, respectively (17, 25) . This may suggest that the combined use of OFLX with multidrug regimens involving KRM may not be advisable, at least when OFLX is administered in small dosages as in the present study.
As shown in Table 2, IFN-γ and GM-CSF alone had insignificant antileprosy activity, indicating that IFN-γ and GM-CSF alone are not sufficiently efficacious in activating host macrophages to exert intense anti-Af. leprae activity. In particular, it appears that IFN-γ may require collaboration with certain other lymphokines to fully potentiate the microbicidal activity of macrophages against M. leprae. Notably, combined administration of KRM with IFN-γ but not with GM-CSF somewhat increased the therapeutic efficacy of KRM against M. leprae in mice, although the increase was not statistically significant. Thus, the efficacy of KRM treatment of leprosy patients may be increased when such chemotherapy is combined with appropriate immunotherapeutic agents, including IFN-γ. This finding suggests that combined immunotherapy may be useful for leprosy patients who are treated with multidrug regimens, including rifamycin derivatives. In addition, this finding indicates that IFN-γ, one of the Thl cytokines participating in the expression of cell-mediated immunity against mycobacterial infections (1, 2, 13-16), also plays an important role in the host resistance to M. leprae infection. This supports the hypothesis of roles of CD4+ and CD8+ T cells (in particular. Type I T cells), which can produce IFN-γ in response to the stimulatory signal of M. leprae antigens (10, 11, 21), in the expression of host resistance against M. leprae infections. Indeed, in the present study we found that CD4+ T cell- and CD8+ T cell-enriched lymphocyte fractions as well as whole lymphocyte fractions obtained from euthymic donor mice confer to recipient athymic mice markedly enhanced resistance to M. leprae infection, even if the cell transfer was performed when the M. leprae infection had already been established.
It is unclear why GM-CSF antagonized the therapeutic activity of KRM against M. leprae infection in athymic nude mice. Both of IFN-γ and GM-CSF are capable of up-regulating the antimycobacterial activity (2, 3, 6, 15, 16) and active oxygen radical production (5, 6, 15) of macrophages. Moreover, their enhancement OF the antimicrobial functions OF macrophages are inhibited by IL-4 (8), and production of them by peripheral blood mononuclear cells is suppressed by IL-10 (24). However, there arc some essential differences between the properties of IFN-γ and GM-CSF, as follows: First, macrophages can produce GM-CSF but not IFN-γ (1) Second, IFN-γ but not GM-CSF can up-regulate macrophage production OF reactive nitrogen intermediates, which are important effectors of the mycobactericidal mechanisms OF macrophages (4, 5). Therefore, the finding of differences between the effects of IFN-γ and GM-CSF in combination with KRM in chemotherapy OF M. leprae infection is not unexpected. Further studies are currently underway concerning the reason why GM-CSF antagonized the therapeutic efficacy OF KRM against M. leprae infections induced in athymic nude mice.
Acknowledgment. This study was supported by the Sasakawa Memorial Health Foundation and, in part, by the U.S.-Japan Cooperative Medical Science Program. We are grateful to Dr. M. Matsuoka for donating M. leprae-infected foot pads and to Kaneka Corporation, Ciba Geigy Co., and Daiichi Pharmaceutical Co. for providing KRM-1648, clofazimine, and ofloxacin, respectively.
REFERENCES
1. BARNES, P. F., MODLIN, R. L., and ELLNER, J.J. Tcell responses and cytokines. In: Tuberculosis: Pathogenesis, Protection, and Control. Bloom, B. R., ed. Washington, D.C.: American Society for Microbiology, 1994, pp. 417-435.
2. BERMUDEZ, L. E. and KAPLAN, G. Recombinant cytokines for controlling mycobacterial infections. Trends Microbiol. 3(1995)22-27.
3. CHAN, J. and KAUFMANN, S. H. E. Immune mechanisms of protection. In: Tuberculosis; Pathogenesis, Protection and Control. Bloom, B. R., ed. Washington, D.C.: American Society for Microbiology, 1994, pp. 389-415.
4. CHAN, J., XING, Y., MAGLIOZZO, R. S. and BLOOM, B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175(1992)1111-1122.
5. DING, A. H., NATHAN, C. F. and STUEHR, D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages; comparison of activating cytokines and evidence for independent production. J. Immunol. 141(1988)2407-2412.
6. GASSON, J. C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood 77(1991)1131-1145.
7. GODFREY, K. Statistics in practice; comparing the means of several groups. New Engl. J. Med. 313(1985)1450-1456.
8. Ho, J. L., HE, S. H., RIOS, M. J. C. and WICK. E. A. Interleukin-4 inhibits human macrophage activation by tumor necrosis factor, granulocytemonocyte colony-stimulating factor, and interIeukin-3 for antileishmanial activity and oxidative burst capacity. J. Infect. Dis. 165(1992)344-351.
9. KATOCH, K., RAMU, G., RAMANATHAN, U., SENGUPTA, U., SREEVASTA, S HARMA, V.D., SHIVANNAVAR, C. T., and KATOCH, V. M . Results of a modified WHO regimen in highly bacilliferous BL/LL patients. Int. J. Lepr. 57(1989)451-457.
10. KAUFMANN, S. H. E. Cell-mediated immunity. In: Leprosy. 2nd edn., Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1994, pp. 157-168.
11. KAUFMANN, S. H. E. CD8+ T lymphocytes in intracellular microbial infections. Immunol. Today 9(1988)168-174.
12. MAW, W. W.. TOMIOKA, H., SATO, K., YAMADA, Y. and SAITO, H. Study on the roles of CD4+ and CD8+ T cells in the expression of host resistance to Mycobacterium leprae infection induced in athymic nude mice. Int. J. Lepr. 63(1995)539-545.
13. MOSMANN, T. R., CHEWINSKI, H., BOND, M. W., GIEDLIN, M. A. and COFFMAN, R. L. TWO types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136(1986)2348-2357.
14. MOSMANN, T. R., and MOORE, K. W. The role of IL-10 in crossregulation of Th1 and Th2 responses. Immunol. Today 12(1991)A49-53.
15. MURRAY, H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann. Intern. Med. 108(1988)595-608.
16. MURRAY, H. W. Gamma interferon, cytokine-induced macrophage activation, and antimicrobial host defense in vitro, in animal models, and in humans. Diagn. Microbiol. Infect. Dis. 13(1990)411-421.
17. NISHINO, T., IKEDA Y, OTSUKI, M., HAYASHI, H. and IMANISHI, R. In vitro and in vivo antimicrobial activities of pazutloxacin, a new synthetic antimicrobial agent. Chemotherapy 43 S-2(1995)54-65.
18. SAITO, H., TOMIOKA, H. and SATO, K. Purification of M. leprae with special reference to the effects of purified M. leprae vaccines on host macrophage cell functions. Jpn. J. Lepr. 56(1987)101-109.
19. SAITO, H., TOMIOKA, H. and SATO, K. In vivo anti -M. leprae activity of benzoxazinorifaniycin, KRM-1648, in combination with other antimicrobials. Proceedings of Twenty-Ninth Joint Conference on Tuberculosis and Leprosy, Washington, D.C.: U.S.-Japan Cooperative Medical Science Program. 1994, pp. 228-233.
20. SAITO, H.. T OMIOKA, H., SATO, K. and DEKIO, S. Therapeutic efficacy of benzoxazinorifamycin, KRM-1648, in combination with other antimicrobials against Mycobacterium leprae infection induced in nude mice. Int. J. Lepr. 62(1994)43-47.
21. SALGAME, P., AISRAMS, J. S., CLAYBERGER, C., GOLDSTEIN, H., CONVIT, J., MODI.IN, R. L. and B LOOM, B . R. Differing lymphokine profiles of functional subsets of human CD4 and CDS T cell clones. Science 254(1991)279-282.
22. SHANNON, E. J., C HEHL, S., JOB, C. K. and HASTINGS, R. C. Adoptively transferred reactivity to M. leprae in nude mice infected with M. leprae. Clin. Exp. Immunol. 70(1987)279-282.
23. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112(1960)445-454.
24. SIBLING, P. A., ABRAMS, J. S., YAMAMURA, M., SALGAME, P., BLOOM, B. R., REA, T. H. and MODLIN, R. L. Immunosuppressive roles for IL-10 and IL-4 in human infection; in vitro modulation of T cell responses in leprosy. J. Immunol. 150(1993)5501-5510.
25. TACHIZAWA, H., TSUMURA, M., UNE, T. and SATO, K. Phase I study on DL-8280. Chemotherapy 32 S-l(1984)118-149.
26. TOMIOKA, H. and SAITO, H. Therapeutic efficacy of some new quinolones and a combination of ofloxacin with rifampin against Mycobacterium leprae infection induced in athymic nude mice. Int. J. Lepr. 61(1993)250-254.
27. TOMIOKA, H. and SAITO, H. In vivo, antileprosy activity of the newly synthesized benzoxazinorifamycin, KRM-1648. Int. J. Lepr. 61(1993)255-258.
28. WHO STUDY GROUP. Chemotherapy of leprosy for control programmed. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. W. W. Maw, M.D.; Department of Microbiology and Immunology, Shimane Medical University, Izumo 693, Japan. H. Saito, M.D., Ph.D., National Institute for Leprosy Research, Tokyo 189, Japan.
2. H. Tomioka, Ph.D.; Department of Microbiology and Immunology, Shimane Medical University, Izumo 693, Japan. H. Saito, M.D., Ph.D., National Institute for Leprosy Research, Tokyo 189, Japan.
3. K. Sato, Ph.D., Department of Microbiology and Immunology, Shimane Medical University, Izumo 693, Japan. H. Saito, M.D., Ph.D., National Institute for Leprosy Research, Tokyo 189, Japan.
Reprint requests to Dr. Tomioka at the above address or FAX 81-853-21-9220.
Received for publication on 10 October 1996.
Accepted for publication in revised form on 19 May 1997.