- Volume 65 , Number 3
- Page: 352–6
M. leprae binds to a 28-30-kDa phosphorylated glycoprotein of rat peripheral nerve
ABSTRACT
To understand Mycobacterium leprae- peripheral nerve interaction, we have investigated the binding of M. leprae to rat peripheral nerve proteins in an in vitro model using 32P-phosphorylated proteins of the peripheral nerve. Intact M. leprae binds to a major phosphorylated protein of 28-30 kDa and, to a minor extent, to a few proteins of molecular weight 45-55 kDa. This binding was more specific for M. leprae since only insignificant binding was observed with other bacteria, such as M. bovis or Escherichia coli. M. leprae did not show binding to several phosphorylated proteins of the rat brain. The 28-30-kDa binding protein of the rat peripheral nerve was found to be a glycoprotein by concanavalin A-Sepharose column chromatography.RÉSUMÉ
Afin de comprendre l'interaction Mycobacterium leprae-neri périphérique, nous avons analysé les liaisons du M. leprae aux protéines des nerfs périphériques du rat dans un modèle in vitro utilisant des protéines des nerfs périphériques phosphorylées au ,:P. Le M. leprae intact se lie à une protéine majeure phosphorylée de 28-30 kDa et, dans une moindre mesure, à quelques protéines de poids moléculaire 45-55 kDa. Cette liaison était plus spécifique pour M. leprae, puisque seules des liaisons non significatives ont été observées avec d'autres bactéries, telles que M. bovis ou Escherichia coli. M. Leprae n'a pas montré qu'il se liait à différentes protéines phosphorylées du cerveau de rat. On a trouvé par chromatographic en colonne sur concanavaline A-Sépharose que la protéine de liaison de 28-20 kDa du nerf périphérique de rat était une glycoprotéine.RESUMEN
Para entender la interacción entre Mycobacterium leprae, y los nervios periféricos, investigamos el enla/.amiento in vitro de M. leprae a proteínas fosforiladas (32P) de nervios periféricos de la rata. El microorganismo intacto se enlaza a una proteína fosforilada de 28-30 kD y en manor grado a algunas proteínas de 45-55 kD. El enlazamiento fue relativamente específico para M. leprae puesto que sólo se observó un enlazamiento insignificante con otras bacterias como M. bovis o Escherichia coli, M. leprae no mostró enlazamiento con varias proteínas fosforiladas del cerebro de la rata. La proteína enlazadora de 28-30 kD de los nervios periféricos fue una glicoproteína que se pudo separar por cromatografía en columna de sefarosa-concanavalina A.Many pathogenic bacteria specifically recognize and bind to the carbohydrate moieties of the host cell surface (8). One of the hallmarks of leprosy is the infection of peripheral nerves by Mycobacterium leprae (9, 10). Recent studies in nasal epithelial cells have shown that M. leprae bind to the extracellular matrix protein fibronectin (FN). This, in turn, binds to integrin FN receptors which help in the internalization of the bacilli (2, 18). However, little is understood about the M. lepra -peripheral nerve protein interaction.
Protein phosphorylation is a post-translational modification of proteins important in signal transduction and is especially prominent in nerve tissues (4,5). Recent studies have shown the involvement of phosphorylation events in the binding of bacteria to the host cells (1, 16, 17).
Earlier studies from our laboratory have shown that purified M. leprae could inhibit the phosphorylation of a 28-30-kDa protein of the rat peripheral nerve and of a 25-kDa protein of the human peripheral nerve (12). One of the mechanisms proposed for the observation was an interaction between M. leprae and the phosphorylated proteins.
In continuation of this observation, we report here that M. leprae binds to the following phosphorylated proteins from the rat peripheral nerve: a) a major 28-30-kDa protein and b) a few minor proteins in the molecular weight range of 45-55 kDa. Further, we have characterized the 28-30-kDa protein as a glycoprotein.
MATERIALS AND METHODS
Tris hydroxymethyl aminomethane (Tris), benzamidine hydrochloride, phenyl methyl sulphonyl fluoride (PMSF) and sodium orthovanadate were obtained from Sigma Chemical Company, St. Louis, Missouri, U.S.A., and gamma 32P ATP was obtained from the Bhaba Atomic Research Centre, Bombay, India. Other reagents used were of analytical grade.
Rat peripheral nerve. Rats (3 months old) were sacrificed by cervical dislocation. The sciatic nerves and brain were dissected and further processed at 4ºC. The tissue was minced and homogenized in Tris HC1 buffer pH 7.6 (10 ml/g tissue), containing 0.001% (w/v) benzamidine hydrochloride, 200 µ M PMSF, 0.1 µ M sodium orthovanadale and 0.1 % (v/v) Triton X-100. The tissue homogenate was centrifuged at 10,000 x g x 30 min in a Sorval refrigerated centrifuge. The supernatant was used for the phosphorylation of proteins. Protein was determined according to Lowry, el al. (14). The rat brain was isolated and processed as described for the peripheral nerve.
Protein phosphorylation. Protein phosphorylation conditions were as standardized earlier in our laboratory (13). The reaction mixture contained 20 µ M Tris-HCl buffer pH 7.5, 20 mM magnesium acetate and gamma 32P-labeled ATP (10 x 107cpm) and 150 µ g of protein in 150 µ l of final volume. The reaction was carried out at 30ºC for 10 min, at the end of which it was either subjected to SDS-gel electrophoresis or cooled on ice and used for binding studies.
Bacilli. M. leprae were isolated from the skin biopsies of borderline lepromatous leprosy patients and from the foot pads of inoculated mice and stored at -20ºC prior to use as described earlier (12). The final bacilli pellet was resuspended in 1 ml of 10 mM phosphate buffered saline (PBS), pH 7.4. containing 0.1 % bovine serum albumin (BSA). Escherichia coli was obtained from cultures provided by the Wellcome Research Laboratory of this Institution. M. bovis was obtained from The Tuberculosis Research Centre. Madras. India. E. coli and M. bovis were heated at 100ºC for 2 min prior to use. Lepromin H was prepared as described earlier (15). The storage of M. leprae and its treatment with NaOH, and heating of E. coli and M. bovis caused the inactivation of any protein kinases and protein phosphatases in these organisms. M. leprae, lepromin H, M. bovis and E. coli, each of 107 bacilli, washed and suspended in PBS containing 0.1% BSA. were used for binding studies.
Binding assay. The binding assay methodology was based upon earlier studies (6, 22). The bacilli suspended in 100-400 µ l PBS containing 0.1% BSA was added to 32p phosphorylated nerve proteins. This was incubated at 4ºC with gentle shaking for 2 hr. To the suspension. 50 volumes of buffer containing 145 mM NaCl. 10 mM Tris HCI. pH 7.2, and 0.1% (v/v) Triton X-100 was added and microfuged for 1 min. Under these conditions the bacilli were sedimented. The pellet was resuspended in PBS and sedimented by centrifugation. This step was repeated 7-10 times at the end of which 100 µ l of SDS dissociation buffer [186 mM Tris HCI buffer. pH 6.8, 6% (v/v) beta-mercaptoethanol. 20% (v/v) glycerol, 6% (w/v) sodium dodecyl sulfate and 0.001% (w/v) bromophenol blue] was added to the pellet and heated at 100ºC for 3 min. Similar incubations were carried out using M. bovis, E. coli and lepromin H. SDS-gel electrophoresis was carried out on 12.5% gels according to Laemmli (11). Further processing and autoradiography of the dried gels were carried out as mentioned earlier (13).
Concanavalin A-Sepharose column chromatography of phosphorylated proteins. Rat peripheral nerve proteins were phosphorylated as mentioned earlier. The phosphorylated proteins (400 µ g) were applied on a column of concanavalin A-Sepharose (0.5 x 3.0 cm) and the glycopro terns were eluted by alpha methyl glucoside. The details of the procedure are as mentioned earlier (13).
Periodic acid-Schiff staining for glycoprotein. Staining for the glycoproteins on gel after SDS-gel electrophoresis of proteins wtis done using the periodate-Schiff reagent according to Segrest and Jackson (19).
RESULTS
An autoradiogram after SDS-gel electrophoresis of the reaction mixtures in which rat peripheral nerve extract was incubated with gamma 32P ATP under the assay conditions showed 32P-labeled proteins of molecular weights ranging from 14-200 kDa (Fig. 1, lane 1).
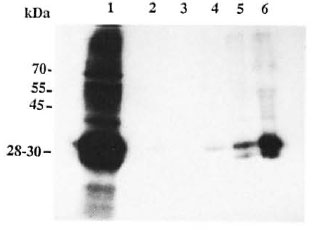
Fig. 1. Autoradiogram showing phosphorylated proteins: Lane I shows 'P-phosphorylated peripheral nerve protein ( 150 µ g) (32P-PNP). The same amount ( 150 µ g) of 32P-PNP was used for the binding assay with each of the bacilli. Lanes 2-5 show the 32P-PNP which are bound to the bacteria (10 bacilli used in each case) alter the binding assay. The binding assay was carried out as described in Materials and Methods. Bacilli used were: Lane 2 = lepromin H; Lane 3 = nil; Lane 4 = E. coli; Lane 5 = M. bovis'. Lane 6 = M. leprae.
The 32P-labeled rat nerve proteins were incubated with M. leprae, and the protein bound to the bacteria was isolated by centrifugation as described in Materials and Methods. This M. leprae-protein complex was subjected to SDS-gel electrophoresis and autoradiography. The autoradiogram showed a major 28-30-kDa protein and minor bands in the range of 45-55 kDa (Fig. 1. lane 6). indicating that these proteins could bind to M. leprae. Similar experiments with M. bovis (Pig. 1. lane 5) showed relatively reduced levels of the 28-30-kDa protein and the other minor bands. E. coli (Fig. 1, lane 4) and lepromin H (Fig. 1, lane 2) showed only insignificant levels of the bound 28-30-kDa protein. There were no32P-labeled proteins in the control tube in which the binding assay was carried out with the 32P-labeled proteins in the absence of bacilli (Fig. 1, lane 3).
Proteins of M. leprae, E. coli and M. bovis did not undergo any phosphorylation as observed in separate control experiments.
When the 32P-labeled proteins of the rat peripheral nerve were passed through the concanavalin A-Sepharose column, the 28-30-kDa protein was found to bind to the column. It could be eluted with alpha methyl glucoside (Fig. 2). The 28-30-kDa protein also stained positive with periodate-Schiff's staining on gel (result not shown).
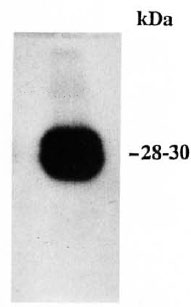
Fig. 2 Autoradiogram showing 32P-phosphorylated 28-30-kDa protein eluted from the concanavalin A-Sepharose column, subjected to SDS-gel electrophoresis (12.5% gel).
Similar experiments were carried out with the rat brain phosphorylated proteins. None of the bacteria used in this study showed any binding to the brain 32P-phosphorylated proteins (results not shown).
DISCUSSION
As mentioned earlier, M. leprae bind to the extracellular matrix protein FN in the nasal mucosal cells and this, in turn, binds to integrin FN receptors which help internalization of the bacilli (2, 18). However, this mechanism does not explain the specificity of the bacilli for the nerve tissue.
To explain the tissue specificity of certain pathogenic bacilli, another type of host pathogen interaction has been described recently wherein pathogenic bacteria bind to specific mammalian cell membrane protein receptors present on target cells. Staphylococcus aureus is found to bind to the 50-kDa protein of endothelial cells (22) and Ureaplasma urealyticum to the 30-kDa, 17-kDa, 16-kDaand 14-kDa proteins of human epithelial cells (21). These studies have shown that the host cell membrane protein receptors play a major role in tissue specificity of bacterial adhesion and invasion.
The 28-30-kDa protein is the major phosphorylated glycoprotein of the rat peripheral nerve eluted from the concanavalin A-Sepharose chromatography column by alpha methyl glucoside. The molecular weight and the phosphorylatable and glycoprotein nature of the protein indicate that it could be the P protein of the peripheral nerve (7). P protein is the major myelin protein of the peripheral nerve and has a significant role to play as a molecular Velcro protein between myelin membranes(3, 20). Phosphorylated brain proteins did not show binding to M. leprae, suggesting that M. leprae has specificity to peripheral nerve tissue protein.
The binding of M. leprae to the major 28-30-kDa protein and a few minor 45-55-kDa proteins was significantly higher compared to that of M. bovis and E. coli. This may be due to the specificity of M. leprae in binding the protein. As observed in our earlier work, the decreased phosphorylation of the 28-30-kDa protein in the presence of M. leprae could be due to the binding of M. leprae to this protein (12).
The present work is focused on the phosphorylated host tissue protein binding to M. leprae. Further experiments are needed to identify the 28-30-kDa phosphoprotein and the nature of the 45-55-kDa phosphorylated proteins which bind to M. leprae, and to know whether any nonphosphorylated proteins of peripheral nerve could bind to M. leprae.
In conclusion, the present investigation shows that intact M. leprae bind to a major 28-30-kDa protein and a few minor 45-55-kDa proteins of the rat peripheral nerve phosphorylated in vitro. This M. leprae-pvo tein interaction could have a role in pathogenesis. The 28-30-kDa protein is the major phosphorylatable glycoprotein of the rat peripheral nerve that resembles the Po protein of the peripheral nerve in its characteristics.
Acknowledgment. LMS and PRS are grateful to the Council of Scientific & Industrial Research, New Delhi, India, for awarding them a research associateship and senior research fellowship, respectively. The work was supported by a grant from the Department of Biotechnology (New Delhi). We are thankful to Mr. Pugazenthi, St. Thomas Hospital, Chettupattu, for technical help.
REFERENCES
1. BERMUDEZ, L. E. and YOUNG, L. S. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect. Immun. 62(1994)2021-2026.
2. BYRD, S. R., GELBER, R. and BERMUDEZ, L. E. Roles of soluble fibronectin and β integrin receptors in the binding of Mycobacterium leprae to nasal epithelial cells. Clin. Immunol. Immunopathol. 69(1993)266-271.
3. CHOE, S. Y Packing of myelin protein P . Neuron 17(1996)363-366.
4. EDELMAN, A. M., BLUEMENTHAL, D. K. and KREBS, E. G . Protein serine/threonine kinases. Ann. Rev. Biochem. 56(1987)567-613.
5. EICHBERG, J. and IYER, S. Phosphorylation of myelin proteins: recent advances. Neurochem. Res. 21(1996)527-535.
6. FLUGEL, A., KOOPS, S. H., HEESEMANN, J., K HUN, K., SOROKIN, L., BURKHARDT, H., VON DER MARK K. and EMMRICH, F. Interaction of enteropathogenic Yersinia enterocolitica with complex basement membranes and the extracellular matrix proteins collagen type IV, laminin-1 and -2, and nidogen/entactin. J. Biol. Chem. 269(1994)29732-29738.
7. HILMI, S., FOURNIER, M., VALEINS, H., GANDER, J. and BENNET, J. Myelin Pti glycoprotein: identification of the phosphorylated site phosphorylated in vitro and in vivo by endogenous protein kinases. J. Neurochem. 64(1995)902-907.
8. ISBERG, R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science 252(1991)934-938.
9. IYER, C. G. S. Predilection of M. leprae for peripheral nerves: neurohistopathologic observations. Int. J. Lepr. 33(1965)634-645.
10. JOB, C. K. Nerve damage in leprosy. Int. J. Lepr. 57(1989)532-539.
11. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227(1970)680-685.
12. LAVANYA, M. S., J OB, C. K. and BALASUBRAMA-NIAN, A. S. Effect of Mycobacterium leprae on peripheral nerve protein phosphorylation-a preliminary study. (Letter) Int. J. Lepr. 64(1996)333-335.
13. LAVANYA, M. S., KORULA, K. J. and BALASUBRA-MANIAN, A. S. Protein phosphorylation in human peripheral nerve: altered phosphorylation of a 25 kDa glycoprotein in leprosy. Neurochem. Res. 21(1996)707-712.
14. LOWRY,O. H, ROSEBROUGH, N. J., FARR, A. L. and RANDALL, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1951)265-275.
15. MEYERS, W. M., WALSH, G. P., B ROWN, H. L., REES, R. J. W. and CONVIT, J. Naturally acquired leprosy-like disease in the nine-banded armadillo ( Dasypus novemcinctus ): reactions in leprosy patients to lepromins prepared from naturally infected armadillos. J. Reticuloendothel. Soc. 22(1977)369-375.
16. MURAKAMI, Y., H ANAZAWA, S., WATANABH, A., NAGANUMA, K., IWASAKA, H, KAWAKAMI, K. and KITANO, S. Porphyromonas gingivalis fimbriae induce 68 kilodalton phosphorylated protein in macrophages. Infect. Immun. 62(1994)5242-5246.
17. ROSENSHINE, I., RUSCHKOWSKI, S., FOUBISTER, V. and FINLAY, B. B. Salmonella typhimurium invasion of epithelial cells: role of induced host tyrosine protein phosphorylation. Infect. Immun. 62(1994)4969-4974.
18. SCHOREY, J. S., LI, Q., MCCOURT, D. W.. BONG-MASTEK, M., CLARK-CURTISS, J. E., RATLIFF, J. L. and BROWN, E. J. A Mycobacterium leprae gene encoding a libronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect. Immun. 63(1995)2652-2657.
19. SEGREST, J. P. and JACKSON, R. L. Molecular weight determination of glycoproteins by Polyacrylamide gel electrophoresis in sodium dodecyl sulphate. Methods Enzymol. 28(1972)54-63.
20. SHAPIRO, L., DOYLE, J. P., HENSLEY, P., COLMAN, D. R. and HENDRICKSON, W. A. Crystal structure of the extracellular domain from Po, the major structural protein of peripheral nerve myelin. Neuron 17(1996)435-450.
21. SMITH, D. G. E., RUSSELL, W. C. and THIRKEL, D. E. Adherence of Ureaplasma urealylicum to human epithelial cells. Microbiology 140(1994)2893-2898.
22. TOMPKINS, D. C, HATCHER, V. B., PATEL, D., ORR, G. A., HIGGINS, LORE L. and LOWY, F. D. Human endothelial cell membrane protein that binds to Staphylococcus aureus in vitro J. Clin. Invest. 85(1990)1248-1254.
1. L. M. Suneetha. M.Sc., Ph.D.; Schieffelin Leprosy Research & Training Centre. Karigiri, Tamil Nadu 632 106, India.
2. P. R. Satish M.Sc.; Schieffelin Leprosy Research & Training Centre. Karigiri, Tamil Nadu 632 106, India.
3. S. Suneetha. M.B.B.S.. D.C.P., Schieffelin Leprosy Research & Training Centre. Karigiri, Tamil Nadu 632 106, India.
4. C. K. Job. M.D.. F.R.C.Path., St. Thomas Hospital and Leprosy Centre. Chettupattu, Tamil Nadu 632 004, India.
5. M.Sc: A. S. Balasubramanian, M.Sc. Ph.D., Neurochemistry Laboratory, Christian Medical College Hospital, Vellore, Tamil Nadu 632 004. India.
Reprint requests to Prof. A. S. Balasubramanian at the above address or Fax 91-416-32035.
Received for publication on 14 February 1997.
Accepted for publication on 31 March 1997.