- Volume 65 , Number 3
- Page: 357–65
A comparison of the expression of NGFr, PGP 9.5 and NSE in cutaneous lesions of patients with early leprosy using immunohistochemistry
ABSTRACT
We examined the immunohistochemical expression of the neuronal proteins NGFr, PGP 9.5, and NSE in cutaneous lesions of patients with early leprosy and in the skin of normal individuals. PGP 9.5- and NSE-immunoreactive nerve fibers were decreased in the skin of leprosy patients. This reduction was topographically unrelated to the early leprosy infiltrate. However, no difference in the expression of NGFr was found between the leprosy patient and normal groups. It was shown that there is a selective alteration in the expression of neuronal proteins in early leprosy lesions which seems to be unrelated to the inflammatory infiltrate in the initial stages of leprosy. Pathogenic mechanisms other than inflammation, which are intrinsic to the Mycobacterium leprae-nevve relationship, may thus contribute to the nerve damage in leprosy neuropathy.RÉSUMÉ
Nous avons examiné l'expression immunohistochimique des protéines neuronales NGFr, PGP 9.5 et NSE dans les lésions cutanées de patients ayant une lèpre débutante et dans la peau d'individus en bonne santé. Les fibres nerveuses immunoréetives PGP 9.5 et NSE étaient en diminution ches les patients lépreux. Cette diminution n'était pas liée topographiquement à l'infriltrat de la lèpre débutante. Cependant, aucune différence dans l'expression de NGFr n'a été observée entre les groupes de patients lépreux et d'individus normaux. On a montré qu'il y a une modification sélective de l'expression des protéines neuronales dans les lésions de lèpre débutante, qui semble non liée à l'infiltrat inflammatoire des stades débutants de la lèpre. Les mécanismes pathogéniques autres que l'inflammation, qui sont intrinsèques à la relation nerf-Mycobacterium leprae, pourraient donc contribuer aux lésions nerveuses dans la neuropathie lépreuse.RESUMEN
Examinamos la expresión inmunohistoquímiea de las proteínas neuronales NGFr, PGP 9.5, y NSE, en las lesiones cutáneas de la lepra temprana y en la piel de individuos sanos. En los pacientes con lepra encontramos una disminución de las libras nerviosas que expresan las proteínas PGP 9.5 y NSE. Esta reducción no estuvo topográficamente relacionada con la localización de los infiltrados tempranos de la lepra. La expresión de la proteína NGFr fue similar entre los pacientes con lepra y los individuos sanos. Demostramos que hay una alteración selectiva en la expresión de las proteínas neuronales en las lesiones de la lepra temprana que no parece ester relacionada con el infiltrado inflamatorio en las etapas tempranas de la lepra. Probablemente otros mecanismos patogénicos, distintos a la inflamación misma, intrínsicos a la relación Mycobacterium leprae-nervio, puedan contribuir al daño en la neuropatía de la lepra.Peripheral neuropathy is the chief cause of the physical deformities and disabilities found in leprosy (3). The peripheral nerves in leprosy are affected by either an epithelioid- or a Mycobacterium leprae- glutted histiocytic infiltrate accompanied by a reduction in the number of nerve libers (10-14). In leprosy, the early pathogenic mechanisms of the nerve lesion have not yet been clarified. Although immunopathogenic processes are likely to be involved in causing leprosy nerve damage (21), Jacobs, et al. (9) and Shetty, et al. (18) do not rule out the existence of a direct pathogenic mechanism in leprosy neuropathy.
Neuron-specific enolase (NSE) and the protein gene product 9.5 (PGP 9.5) are widely utilized as neuronal markers in studies on peripheral nerve pathologies (11-12). These studies have shown the extent of peripheral nerve damage in target organs in diseases in which the peripheral nervous system was involved.
The NGF family of neurotrophins binds to the nerve growth factor receptor (NGFr)(13). It seems that the study of NGFr expression might be a good way to approach the investigation of the functions of the peripheral nervous system (6). To date, neurotrophism in leprosy has only been studied by Anand, et al. (1), who detected depletion of NGF in the skin of leprosy patients by an enzyme-linked immunoassay (ELISA). Attempts to detect the existence of neurotrophic disturbances by examining the immunohistochemical expression of NGFr were carried out in peripheral neuropathies other than leprosy (17-19) and in amyotrophic lateral sclerosis (2).
The search for an early pathogenesis of leprosy neuropathy is warranted by the need to prevent the severe disabilities present in the advanced stages of the disease. The immunohistochemical expression of the neuronal proteins PGP 9.5 and NSE, as well as the the low-affinity NGFr expression, as a preliminary approach to neurotrophism in leprosy were investigated in order to detect early alterations of neurons and the relationship of these changes to the initial restricted leprosy infiltrate.
According to Gupte (8) early leprosy is characterized by the presence of at least two of the following signs: macular lesions, sensory disturbances, nerve thickening and positivity for acid-fast bacilli (AFB). We were also interested in the NGFr expression as a preliminary approach to neurotrophism in leprosy.
MATERIALS AND METHODS
Eleven biopsies of 11 untreated early leprosy patients (Table 1) and samples of corresponding normal skin areas from 7 plastic surgery patients and 2 volunteers, for utilization as an age- and sex-matched control group, were selected for this study.
The sensory function of the skin was evaluated according to the responses of the patients to different sensory stimuli. Tubes filled with warmed or cold water, a swab of cotton, and a pin were utilized as stimuli for testing thermal, touch and pain sensitivity, respectively. All of the patients showed abnormal responses to these tests. Histamine was injected into the lesion to test the functional integrity of nerve terminals. Normally, histamine induces the formation of a reddish papule and an erythematous halo at the site of injection. However, none of these patients showed these expected responses. Three individuals had AFB-positive skin smears, but AFB were found only in the biopsy specimens of two patients.
Histopathological findings [hematoxylin and eosin (H&E) and Wade stains] showed a mononuclear inflammatory infiltrate adjacent to the adnexial structures and to the nerve branches of the dermis. In addition to the perineural mononuclear cell infiltrate (MCI), seven patients had a single, immature epithelioid granuloma in the dermis close to the vascular structures.
All patients who had a negative AFB test were suspected to have the disease, taking into account the clinical manifestation, the abnormal response to the histamine test, and the presence of perineural and periadnexia! inflammatory infiltrate in the histopathological examination. At the end of multidrug therapy, the diagnosis of leprosy was felt to be confirmed in all of the patients based on the total remission of all pre-existent lesions and neural symptoms.
The skin fragments were fixed in a saturated solution of picric acid in 10% formalin for 2 hr at 4ºC and rinsed in phosphate buffered saline (PBS) to which 10% sucrose was added. They were snap-frozen in CO2, cut as 14- µ m-thick sections, using a Microm cryostat (Heidelberg, Germany), and thawed onto glass slides.
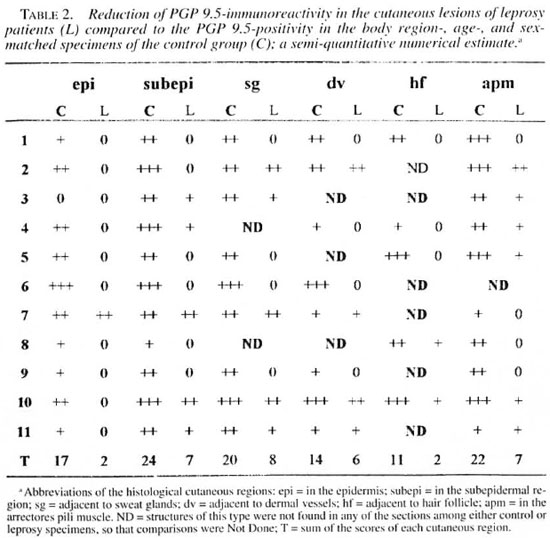
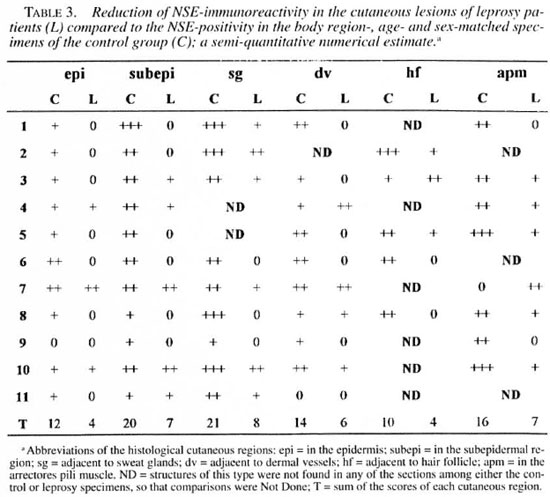
An indirect immunofluorescence investigation (4) was performed using rabbit antihuman PGP 9.5 (1:2000; UltraClone, Cambridge, U.K.), rabbit anti-human NSE (1:800; UltraClone) and mouse anti-human low-affinity NGFr (1:100, Amersham Sweden AB, Solna, Sweden) as primary antibodies. The secondary antibodies used were rhodamine-conjugated goat anti-rabbit immunoglobulin (1:80; Boehringer, Mannheim, Germany) and rhodamine-conjugated goat anti-mouse immunoglobulin (1:80; Boehringer), respectively. The slides were mounted on buffered glycerine, with p-phenylenediamine (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) anti-fading (15), and were observed in a Nikon photomicroscope (Microphot-FXA). Control experiments were performed by using blocked primary antibodies. The secondary antibodies were controlled by omitting the primary ones. The amounts of immunoreactive nerve fibers were determined according to the semiquantitative numerical estimates in the skin of both groups. The numbers of positive fibers were shown as follows: few (+), average (++), and many (+++)- The sections were examined by two investigators.
RESULT
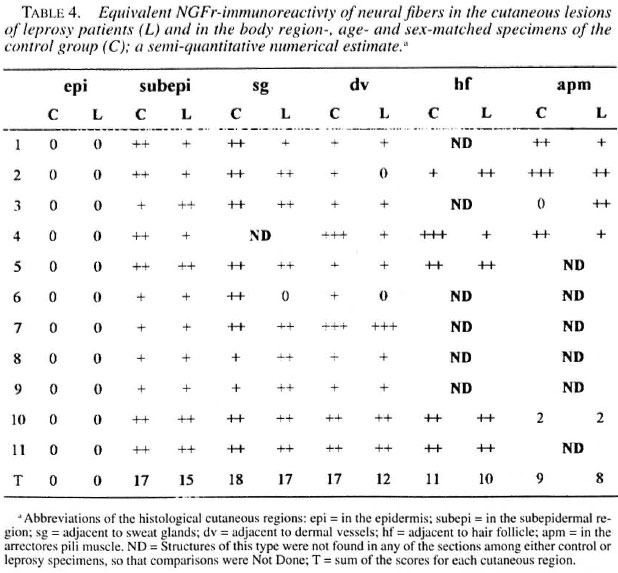
Significant reductions in the amounts of PGP 9.5- and NSE-immunoreactive nerve fibers were found in all regions of the leprosy biopsies, which did not occur in the correspondent regions of the normal specimens (Figs, la, lb; 2a, 2b; Tables 2 and 3). This reduction was also seen in the epidermis in the regions adjacent to adnexial structures and in the interstitium of arrectores pili muscles. However, both control and pathological groups displayed the same NGFr-positivity pattern (Figs. 3a, 3b; Table 4). Moreover, immediately adjacent sections of the same leprosy specimen were NGFr-positive (Fig. 4a), NSE-negative (Fig. 4b), and PGP 9.5-negative. Furthermore, NGFr-positive basai epidermal cells (Figs. 3a, 3b), were observed in both groups, but with a somewhat reduced staining intensity in the leprosy group.
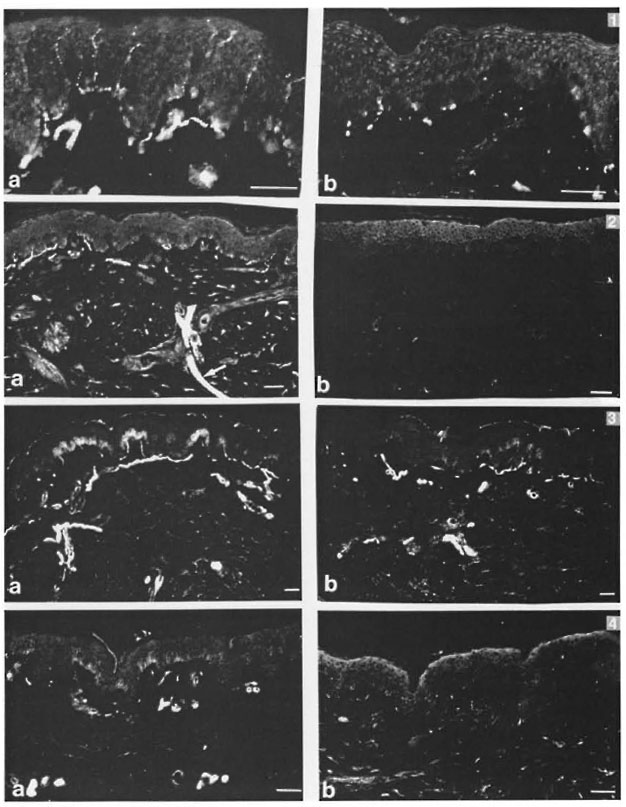
Fig. 1. A greater number of PGP 9.5-immunoreactive nerve libers in the control (a, +++) than in the leprosy skin (b, 0) (indirect immunofluorescence: scale bar = 50 µm).
Fig. 2. A greater number of NSE-immunoreactive nerve libers in the control (a, ++) than in the leprosy skin (b, 0). A long and thick segment of a cutaneous nerve is also seen in (a) (arrow) (indirect immunofluorescence; scale bar = 50 µm).
Fig. 3. Equivalent patterns of NGFr-immunoreactivity in the control (a. +++) and in the leprosy skin fragment (b, +++). There is a reduced staining intensity of basal epidermal cells in the leprosy specimen (indirect immunofluorescence; scale bar = 50 µ m).
Fig. 4. NGFr positively (a. ++) and NSE negativity (b. 0) in immediately adjacent sections of a leprosy specimen. 'I'he weaker positive signals in b are not specific and represent auto-lluorescent dermal elastic libers (indirect immunofluorescence; scale bar = 50 µ m).
DISCUSSION
Extensive dermal regions with reduced numbers of PGP 9.5- and NSE-immunoreactive nerve fibers were only sparsely occupied by a few inflammatory cells encircling adnexial structures and nerve branches (the inflammatory infiltrate occupied much less than one tenth of the biopsy sections). Selective alterations in the expression of neuronal proteins, unrelated to the regionally restricted and incipient early leprosy infiltrate were found in this study. In addition to corroborating Karanth, et al. 's findings (12) in regard to the negativity of these protein expressions, PGP 9.5- and NSE-negativity in the early leprosy lesions do not necessarily indicate absence of nerve fibers, as demonstrated by the NGFr-positive expres-sion coupled with PGP 9.5- and NSE-nega-ti vity in the immediately adjacent sectionsof. the leprosy specimen.
The lack of a topographical relationshipbetween the extensive dermal regions devoid of immunoreactivie tibers and the restriction of the inflammatory infiltrates toadnexial structures and nerve branches suggests the existence of a pathogenic mechanisn in the early stztges of leprosy neuropathy which may act relatix'ely independent ofthe specific inflanunatory process. Pathogenetic mechanisms of leprosy neuropathy other than the immunoinilammatory response occurring in the nerve have beensuspected by Jacobs, et al. (9) and Shetty, et al .(18). These authors have reported axonalatrophy, subperineurial edema, and paranodal demyelination ia the peripheral nervesof leprosy patients. Axonal atrophy and subperineurial edema were detecteil through tiltrastructural examination of nerves whichhad a normal appearance by light microscopy. However, these tindings have not yet been completely explained (9). The para-nodal demyelination was detecteid throughthe examination of teased axons from pripheral nerves (9), and was not considered to result from an auto-immune response. Hypothetically, the axonal atrophy reported by Shetty, et al. (18) may reflect a decrease in the expression of neuronal enzymes, such as PGP 9.5 and NSE, in addition to other proteins. The role of the immunoinflammatory mechanisms in causing late nerve destruction in leprosy cannot be disregarded; however, it is important to identify the critical factor that induces mononuclear cell migration into the nerve. This critical event may arise either from altered nerves or from the bacterial etiologic agent. The blood-nerve barrier must be damaged in the early stages of leprosy to allow for infiltration by leukocytes. These cells are not found in a normal endoneurial compartment (19). The events occurring upon the arrival of bacteria to the nerves up to the initiation of the specific immunoinflammatory response require further investigation.
M. leprae itself could cause the early biochemical and molecular alterations of the nerve biology in leprosy. It is the only bacterium able to infiltrate the normal peripheral nerve. Although it does not have the virulent properties of M. tuberculosis, it may be regarded as a microorganism capable of interacting with the peripheral nerve components and of changing their metabolic pathways. The M. leprae- nerve relationship would be a fascinating subject for future research in leprous neuropathy.
In regard to neurotrophism, no specific leprosy alteration was detected. However, this does not rule out the possibility of neurotrophic disturbances in leprosy, since there are other types of neurotrophic factor receptors with their respective ligands which were not investigated in this study (13). Anand, et al. 's report (1) indicates the need for more refined biochemical methods for the detection of nerve growth factors and their receptors in the tissues.
Our findings demonstrate the existence of a selective alteration of neuronal protein expression in the cutaneous innervation, which may or may not be directly related to the inflammatory infiltrate of early leprosy.
Acknowledgment. This study was supported by grants from the CNPq (Brazil) and from the Karolinska Institute. For a generous supply of antisera, we are grateful to Professor Lars Olson, Department of Neuroscience, Karolinska Institute, Stockholm, Sweden. We also express our thanks to the staffs of the following institutions: Leprosy Outpatient Service, Oswaldo Cruz Foundation, and the Department of Internal Medicine (Dermatology Unit), Department of Pathology and Department of Plastic Surgery, Antonio Pedro Hospital, Fluminense Federal University, Niterói, Brazil. Ms Eva-Karin Johansson is warmly acknowledged for expert secretarial assistance.
REFERENCES
1. ANAND, P., PANDYA, S., LADIWALA, U., SINGHAL, B., SINICROPI, D. V. and WILLIAMS-CHESTNUT, R. E. Depletion of nerve growth factor in leprosy. Lancet 344(1994)129-130.
2. AQUILONIUS, S. M., ASHMARK, H., EBENDAL, T. and GILLBERG, P. G. No re-expression of high affinity nerve growth factor binding sites in spinal motor neurons in amyotrophic lateral sclerosis. Eur. Neurol. 32(1992)216-218.
3. BRYCESON, A. and PFALTZGRAFF, R. E. Complications due to nerve damage. In: Leprosy. 6th edn. Bryceson. A. and Pfaltzgraff, R. E., eds. Edinburgh: Churchill Livingston, 1990, pp. 133-152.
4. COONS, A. H. Fluorescent antibody methods. In: General Cytochemical Methods. Vol. 1. Danielli, J. F., ed. New York: Academic Press, 1958, pp. 399-422.
5. DASTUR, D. K., RAMAMOHAN, Y. and SHAH, J. S. Ultrastructure of lepromatous nerves. Neural pathogenesis in leprosy. Int. J. Lepr. 41(1973)47-80.
6. DAVIES, A. M., BANDTI.OW, C, HEUMANN, R., KORSCHING, S., ROHRER, H. and THOENEN, H. Timing and site of nerve growth factor sysnthesis in developing skin in relation to innervation and expression of the receptor. Nature 326(1987)353-358.
7. GIBBELS, E., HENKE-LUBKE, O. and KLINGMOLLER, G. Unmyelinated nerve fibres in leprosy; a qualitative study of sural nerve biopsies in 2 cases of lepromatous leprosy. Lepr. Rev. 59(1988)153-162.
8. GUPTE, M. D. Early diagnosis of leprosy under held conditions. Lepr. Rev. 65(1993)3-12.
9. JACOBS, J. M., SHITTY, V. P. and ANTIA, N. H. Teased fibre studies in leprous peripheral neuropathy. J. Neurol. Sci. 79(1987)301-313.
10. JOB, C. K. and DESIKAN, K. V. Pathologic changes and their distribution in peripheral nerves in lepromatous leprosy. Int. J. Lepr. 36(1968)257-270.
11. JOHANSSON, O., HILLIGES, M. and STAHLE-BACK-DAHL, M . Intraepidermal neurorn-specilic enolase (NSE)-immunoreactive nerve fibres: evidence for sprouting in uremic patients on maintenance hemodialysis. Neurosci. Lett. 99(1989)281-286.
12. KARANTH, S. S., SPRINOALL, D. R., LUCAS, S., LEVY, D., ASHBY, P., LEVENE, M. M. and POLAK, J. M. Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J. Pathol. 157(1989)15-26.
13. MEAKIN, S. O. and SHOOTER, E. M . The nerve growth factor family of receptors. Trends Neurosci. 15(1992)323-331.
14. MEHTA, L. N., SHETTY, V. P., ANTIA, N. H. and IRANI, P. F. Quantitative histologic and ultrastructural studies of the index branch of the radial cutaneous nerve and its correlation with electrophysiologic study. Int. J. Lepr. 43(1975)256-264.
15. PLATT, J. L. and MICHAEL, A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J. Histochem. Cytochem. 31(1983)840-842.
16. PROPERZI, G., FRANCAVILLA, S., POCCIA, G., ALOISI, P., GU, X. H., TERENGHI, G. and POLAK, J. M. Early increase precedes a depletion of VIP and PGP 9.5 in the skin of insulin-dependent diabetics; correlation between quantitative imrnunohistochemistry and clinical assessment of peripheral neuropathy. J. Pathol. 169(1993)269-277.
17. SCIACCO, M., SCARPINI, E., B ARON, P. L., DORONZO, R., MOGGIO, M., PASSERINE D. and SCARLATO, G. Sural nerve immunoreactivity for nerve growth factor receptor in a case of localized hypertrophic neuropathy. Acta. Neuropathol. (Berl.) 83(1992)547-553.
18. SHETTY, V. P., ANTIA, N. H. and JACOBS, J. M. The pathology of early leprous neuropathy. J. Neurol. Sci. 88(1988)115-131.
19. SOBUE, G, YASUDA, T, MITSUMA, T, ROSS, A. H. and PLEASURE, D. Expression of nerve growth 9.5 and NSE in Early Lesions factor receptor in human peripheral neuropathies. Ann. Neurol. 24(1988)54-72.
20. STONER, G. L. Hypothesis: importance of the neural predilection of Mycobacterium leprae in leprosy. Lancet 2(1979)994-996.
21. TURK, J. L., CURTIS, J. and DE BLAQUIÈRE, G. Immunopathology of nerve involvement in leprosy. Lepr. Rev. 64(1993)1-6.
1. M.D., Experimental Dermatology Unit, Department of Neuroscience, Karolinska Institute, Stockholm, Sweden and Laboratory of Leprosy, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil.
2. M.D., Laboratory of Leprosy, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil.
3. Technician; Experimental Dermatology Unit, Department of Neuroscience, Karolinska Institute, 171 77 Stockholm, Sweden.
4. Ph.D., Experimental Dermatology Unit, Department of Neuroscience, Karolinska Institute, 171 77 Stockholm, Sweden.
Reprint requests to Dr. Johansson.
Received for publication on 29 July 1996.
Accepted for publication in revised form on 25 March 1997.