- Volume 65 , Number 4
- Page: 450–5
Risk factors for type 1 reactions in leprosy
ABSTRACT
A cohort of new borderline leprosy patients seen over a 7-year period were examined retrospectively for risk of type 1 reactions (T1R) associated with 12 clinical and laboratory parameters. Logistic regression analysis was used to identify a strong link between facial patches and cutaneous T1R and enlarged ulnar nerves and neural T1R. Anti-phenolic glycolipid-I seropositivity, a positive bacterial index, and disease in more than two body areas were also identified as risk factors for T1R. These data indicate that there are important clinical data which can be used to predict an individual patient's risk of developing T1R.RÉSUMÉ
Une cohorte de nouveaux patients borderline vus au cours d'une période de 7 ans a été examinée rétrospectivement quant à leur risque de développer une réaction de type 1. associé à 12 paramètres cliniques et de laboratoire. On a réalisé une analyse par régression logistique pour identifier un lien important entre des taches sur le visage et une réaction de type 1 cutanée, et des nerfs cubitaux épaissis et une réaction de type 1 neurologique. La séropositivité vis-à-vis du glycolipide phénolique I, un indice bactérien positif, et une maladie disséminée dans plus de deux paities du corps ont aussi été identifiés comme des facteurs de risque pour les réactions de type 1. Ces données indiquent qu'il y a des éléments cliniques importants qui peuvent être utilisés pour prédire le risque individual d'un patient de développer une réaction de type 1.RESUMEN
Se hizo un análisis retrospectivo de los expedientes correspondientes a un grupo de pacientes con lepra intermedia observados durante un periodo de 7 años, para establecer el riesgo de presentar reacciones del tipo 1 (RTI). Se tomaron en cuenta 12 parámetros clínicos y de laboratorio y se usaron análisis de regresión logística pare calcular la correlación entre la presencia de manchas faciales y las reacciones RTI de la piel y la correlación entre la presencia de nerviosulnares engrosados y las R'I'l neurales. Entre los factores de riesgo se identificaron la presencia de anticuerpos anti-gliculípido fenóiico I. los indices bacterianos positivos y la presencia de lesiones en más de 2 áreas del cuerpo. Fistos datos indican que existen importantes datos clínicos que pueden usarse para predecir el riesgo de un paciente en particular de desarrollar una RT1.Type 1 reactions (T1R) are important pathological events in borderline leprosy patients. Approximately one-third of borderline leprosy patients will have a T1R before, during or after treatment. T1R presents as a spectrum of symptoms involving the skin, peripheral nerves or both. The prevention of disability due to nerve damage sustained in T1R has motivated numerous workers to seek to define risk factors for T1R in various studies. A number of factors including vaccination, chemotherapy, pregnancy, facial involvement and seropositivity for anti-phenolic glycolipid-I (PGL-I) antibodies have been identified.(3) However, most of the studies have been case reports, and few of these studies have examined the relative importance of clinical and laboratory measurements in the same group of patients. Moreover, comparison between studies has been confounded by the varying definitions of T1R used.
In the present study, we have used a comprehensive definition of T1R in a retrospective cohort of 534 previously untreated, borderline leprosy patients seen over 7 years at Anandaban Leprosy Hospital, Kathmandu, Nepal. We have examined the contributions of 12 clinical and laboratory measurements of the risk of developing neural or cutaneous T1R in patients with single or multiple T1R episodes, in those presenting with T1R at first consultation, and in those in whom T1R develops later during treatment. The aim of this work was to use clinical and laboratory parameters to identify patients who are at high risk of developing T1R and who would benefit from closer supervision and health education.
MATERIALS AND METHODS
We included in this study 658 previously untreated, borderline leprosy (BT, BB, BL) patients registering as regular multidrug therapy (MDT) patients at Anandaban Hospital between 1989 and 1996.
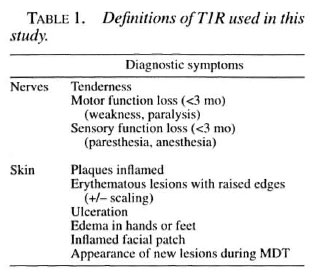
Type I reactions were defined as cutaneous or neural according to the criteria shown in Table 1. Neural T1R was defined as nerve tenderness, motor function loss (weakness or paralysis), or sensory function loss (paresthesia or anesthesia) for less than 3 months. Cutaneous T1R was defined as erythematous lesions with raised edges, inflammation of plaques, ulceration of lesions, edema in the hands or feet, or the appearance of new lesions during therapy.
All data were collected at the patient's first consultation. This included age, sex, and leprosy classification based on the Ridley-Jopling classification (4) and confirmed by histopathology in about half of the cases. The number of years since the appearance of the first symptoms as reported by the patient was recorded as a measure of the length of untreated disease. The cumulative disability index (DI) of each patient according to the World Health Organization (WHO) classification system (12) was also recorded. In addition, the number of body areas with disease were calculated. This was based on the number of body areas with either skin patches or enlarged nerves based on a scale with a maximum of nine, consisting of four for the trunk divided sagitally anterior and posterior, four for the limbs and one for the head (10).
The total number of enlarged nerves was also recorded and enlargement of the ulnar or common peroneal (lateral popliteal) was also noted. Facial involvement was also noted as a facial patch, infiltration, facial paralysis or lagopthalmus.
The Mitsuda lepromin reaction to lepromin A at 28 days after inoculation was measured. Reactions greater than 3 mm were considered positive.
The mean bacterial index (BI) was calculated from slit-skin smears that were taken from a minimum of four sites, including a clinical lesion.
IgM anti-PGL-I antibodies were measured as described previously (5).
Statistics. Differences in the range of variables between different patient groups were tested by means of the Mann-Whitney U test.
Chi-squared contingency tables were used to calculate the unadjusted odds ratios (OR) and 95% confidence intervals (95% CI) for differences in the prevalence of T1R reactions between patients with or without individual clinical or laboratory parameters.
Adjusted OR and 95% CI were calculated by logistic regression to assess the significance of individual factors. All risk factors included in the logistic regression model were known risk factors for Tl R. On analysis, there was colinearity between the leprosy classes (BT, BB, BL) in this data set, so that inclusion of leprosy classes as risk factors was not possible. Models, including each leprosy class individually, were compared to a model that excluded leprosy class altogether. The OR obtained with and without leprosy class included in the model were no more than 10% different. Thus, the adjusted OR are presented from the logistic regression model excluding leprosy class (i.e., with 12 variables).
Because this cohort of patients showed an increase in the prevalence of T1R over time, we investigated the effect of the year of enrollment as a variable in the logistic regression model. The year of enrollment did not emerge as a significant risk factor, and inclusion of this data did not significantly alter the OR for the strongest risk factors. Thus, year of enrollment was not included in the logistic regression analysis, and the risk factors identified were valid over the 7-year intake period.
The risks associated with the clinical parameters collected were calculated for any T1R, T1R at presentation or later in treatment, single or multiple episodes of T1R, and for T1R occurring in either the skin or nerves.
RESULTS
One-hundred-twenty-four patients were excluded from the study for the following reasons: defaulting from treatment (N = 58), transfer to health post (N = 47), evidence of previous treatment (N = 6), or incorrect diagnosis or classification (N = 13). The records of the remaining 534 patients were examined for baseline (i.e., at diagnosis) clinical, bacteriological and serological data as well as history of T1R.
The 534 patients consisted of 361 male and 173 female patients (age range of 7 to 80 years). There were 250 borderline tuberculoid (BT), 57 midborderline (BB) and 227 borderline lepromatous (BL) patients; 91 received WHO/PB-MDT treatment and 441 received WHO/MB-MDT. One patient was treated with dapsone and clofazimine alone because of rifampin sensitivity and one with rifampin and clofazimine due to dapsone sensitivity. The patients were seen regularly at hospital clinics for an average of 16.8 months (range 6-79 months) and to the completion of chemotherapy in 256 cases.
There was facial involvement in 372 patients, consisting of patches (N = 288), infiltration (N = 117), paralysis (N = 5), lagopthalmus (N = 11), other (N = 6). There were an average of 5.6 body areas with disease (range 1-9) and an average of 3.7 enlarged nerves (range 0-13) in the total patient group. Ulnar nerves were enlarged in 361 patients; in 268 of these both ulnar nerves were enlarged. The lateral popliteal was enlarged in 349 patients and in 257 both lateral popliteal nerves were enlarged. Two-hundred-forty-eight patients were skin-smear positive and 286 were skinsmear negative. Lepromin reactions were positive (>3 mm) in 227 patients, negative in 135, and not done in 172. PGI-I antibodies were positive in 286, negative in 231, and not done in 17 cases.
Among the 534 patients, 279 (52%) had T1R during the study period; 213 patients had a single episode, 40 had two, 19 had three, 6 had four, and 1 patient had five episodes of T1R. Thus, there were a total of 379 reactional episodes in this study population. Reactions were classed as skin (127), nerves (48), or both (104) (Table 1). One-hundred-eighty-seven (67%) of T1R episodes were observed in new patients at the first examination and 92 (33%) after the commencement of treatment; 88% of all reactions occurred within the first 6 months of commencing therapy. The frequency of T1R in the different classes of leprosy was significantly higher in BB patients (40/57, 70%, p <0.01) and BL patients (151/227, 66%, p < 0.001) when compared with BT patients (88/250, 35%).
Previous workers (9) have commented on the differences in the timing of T1R in different leprosy classes. The time and type of the reactions in the different leprosy classes were analyzed, but no significant difference could be found. In all of the leprosy classes, the majority of T1R occurred at first presentation or within the first 6 months of treatment. The frequency of neural or cutaneous T1R was not significantly different between BT and BL patients although BB patients had fewer neural T1R cases. A significant difference was noted in the time of occurrence of neural compared with cutaneous T1R. Whereas only 7% of cutaneous T1R episodes occurred after 6 months of treatment, 32% of neural T1R episodes occurred later than 6 months after commencement of MDT (chi-squared = 10.7, p <0.001 ).
Patients with T1R had more diffuse disease than patients without T1R. Thus, the numbers of body areas with disease, enlarged nerves, positive smears and anti-PGL-I seropositives at diagnosis were higher in patients who developed T1R than in those who did not. Conversely, lepromin reaction diameters were significantly smaller in T1R patients than in non-T1R patients (Table 2).
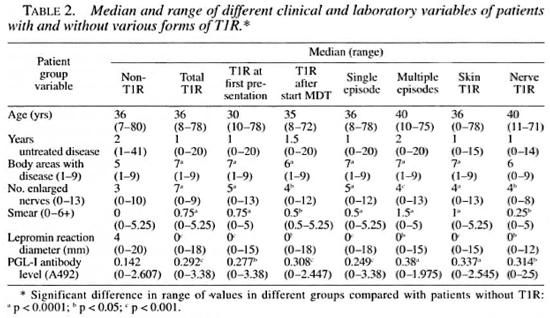
Adjusted OR for 12 clinical factors and various forms of T1R, calculated by logistic regression, are shown in Table 3. Facial involvement was a significant risk factor for T1R and was especially common among patients with T1R at first presentation. This high OR may reflect an ascertainment bias since an inflamed facial patch is a strong motive to seek medical attention and is unlikely to be overlooked by clinicians. Ascertainment bias may also explain the association of a short period of untreated disease with T1R on first presentation. Facial involvement is particularly associated with cutaneous but not neural T1R and is a high risk factor for multiple episodes of T1R.
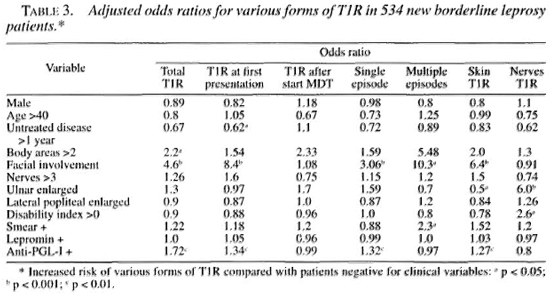
If more than two body areas had disease, this was associated with a higher risk of T1R and with multiple episodes of T1R (although the latter was not statistically significant).
More than three enlarged nerves was not associated with an increased risk of T1R. However, an enlarged ulnar nerve, although not an enlarged lateral popliteal nerve, was strongly associated with episodes of neural T1R and negatively with cutaneous T1R. Patients with established disability (WHO grade >0) were also at increased risk of neural T1R. Patients with a positive skin smear were at higher risk of multiple episodes of Tl R. Patients with antibodies to PGL-I were at increased risk of T1R, especially at first presentation. However, in contrast to previous findings (8), lepromin positivity was not associated with an increased risk of any form of TlR.
DISCUSSION
This group of borderline leprosy patients had a prevalence of T1R of 52% over the average 17 months of observation. This high prevalence is explained by the study population being drawn from a leprosy referral hospital. Field-based studies suggest that T1R occurs in approximately 30% of borderline patients (3). T1R was the presenting symptom in a high proportion of our cases (N = 187, 35% of all patients, and 67% of all T1R). BT patients had a significantly lower prevalence of T1R than did BB/BL patients. The use of clinical classification as a risk factor for T1R may suffer from observer bias due to variability in clinical classifications between different leprosy programs.
Definitions of T1R vary widely. We chose a comprehensive definition, based on a consensus of senior leprologists (Table 1; Waters, personal communication), which clearly defines the cutaneous and neural symptoms of T1R. Our previous study (8) focused on neural symptoms with or without cutaneous involvement. Another study from Nepal (11) used a definition of cutaneous involvement with or without neural involvement and found a difference in risk factors. The breakdown of risk factors by different outcomes (Table 3) shows that different risk factors are associated with neural T1R and cutaneous T1R.
The appearance of a raised erythematous lesion on the face is a strong motive for the patient to seek medical attention and is immediately recognized by the leprosy worker as T1R. The association with T1R may be due to an ascertainment bias, in that these patients are over-represented among new cases. A review of 1226 PB leprosy patients in India (2) revealed a significant relationship between the presence of facial patches and T1R and resultant lagopthalmus, but in less than 10% of patients.
van Brakel, et al. (11) showed an association between the number of body areas with disease (either enlarged nerves or patches) and the risk of T1R. In this group of patients more than two body areas with disease was associated with a twofold increase in risk of T1R and in a higher risk of multiple episodes of T1R.
As in our previous study, the presence of IgM anti-PGL-I antibodies is an independent risk factor for the development of T1R. PGL-I is a product of viable Mycobacterium leprae (6) and antibody levels fall with chemotherapy (7). PGL-I is not a significant target for an inflammatory cellmediated immune response, which is thought to be the pathological basis of T1R ('). However, its association with M. leprae viability makes it a valuable marker of patients with a significant viable bacterial load. The immune target(s) associated with T1R would seem to be associated with live bacteria, a hypothesis supported by the observation in this study as in others that 88% of T1R episodes occur in untreated patients or within the first 6 months of therapy. Although anti-PGL-I antibodies are associated with a positive bacterial index, the bacterial index and anti-PGL-I positivity were independent risk factors for T1R. Skin-smear positivity was associated with an increased risk of multiple episodes of T1R.
By contrast with our previous study, lepromin reactivity was not associated with the risk of developing T1R. The high incidence of T1R in lepromin-negative BB/BL patients in this second and larger study reveals the lepromin reaction to have little or no utility in defining an at-risk patient population.
Neural damage by inflammatory events such as T1R are an important cause of the disability and deformity associated with leprosy. Enlarged ulnar nerves are found in 42% and enlarged lateral popliteal nerves in 36% of all new patients presenting at Anandaban (Lincoln, unpublished observations). Enlargement of either or both nerves have been previously described as risk factors for T1R (9). In this patient group, the enlargement of the ulnar was the strongest predictor of a T1R in nerves. Patients with established disability at presentation were also at increased risk of neural T1R as well. This possibly reflects the susceptibility of the damaged nerve to further inflammatory damage. The period of untreated disease was not associated with an increased risk of T1R in contrast with previous reports of patients with established disease being at higher risk than new patients (3).
In conclusion, T1R which presents as a range of symptoms in the skin and nerves has a range of identifiable risk factors which can be defined by well-established methods. The association of different clinical symptoms with various forms and frequencies of T1R reflects the underlying biology of these inflammatory events, and can serve as a guide to clinicians as to the risk of T1R in individual patient faces.
The definition of these high-risk groups has important implications for the future education of health workers in leprosy and for patient education as well. Interventions in such high-risk groups with prophylactic antiinflammatory drugs at low doses may be an important preventive measure against the pathology associated with these episodes.
Acknowledgment. This work was supported by The Leprosy Mission International. The authors would like to thank the staff and patients of Anandaban Hospital for their cooperation in this study.
REFERENCES
1. COOPER, C. L., MUKLLER, C , SINCHAISRI, T.-A., PIRMEZ, C, CHAN, J., KAPLAN, G.. YOUNG, S. M. M.; WEISSMAN, I. L., BLOOM, B. R., REA, T. H. and MODI,IN, R. L. Analysis of naturally occuring delayed-type hypersensitivity reactions in leprosy by in situ hybridisation. J. Exp. Med. 169 (1989) 1565-1581.
2. HOGEWEG, M., KIKAN, K. U. and SUNEETHA, S. The significance of facial patches and type 1 reaction for the development of facial nerve damage in leprosy; a retrospective study among 1226 paucibacillary leprosy patients. Lepr. Rev. 62 (1991) 143-149.
3. LlENHARDT, C. and FINE, P. E. M. Type 1 reaction, neuritis and disability in leprosy: what is the current epidemiological situation? Lepr. Rev. 65 (1994)9-33.
4. RlDLEY, D. S. and IOPLINO, W. II. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34 (1966) 255-273.
5. ROCHE, P. W., BRITTON, W. J.; FAILBUS, S. S., LUD-WIG, II., THEUVENET, W. J. and ADIGA, R. B. Heterogeneity of serological responses in paucibacillary leprosy: differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int. J. Lepr. 58 (1990) 319-327.
6. ROCHE, P. W.. BRITTON, W. J.; NEUPANE, K. D., FAILBUS, S. S., CHO, S.-N. and THEUVENET, W. J. The response to chemotherapy of serum Mycobacterium leprae-speciñe antigen in multibacillary leprosy patients. Am. J. Trop. Med. Hyg. 44 (1991)702-708.
7. ROCHE, P. W.; FAILBUS, S. S.. NEUPANE, K. D., THEUVENET, W. J. and BRITTON, W. J. Serological monitoring of the response to chemotherapy in leprosy patients. Int. J. Lepr. 61 (1993) 35-43.
8. ROCHE, P. W., THEUVENET, W. J. and BRITTON, W. J. Risk factors for type-1 reactions in borderline leprosy patients. Lancet 338 (1991) 654-657.
9. ROSE, P. and WATERS, M. F. R. Reversal reactions in leprosy and their management. Lepr. Rev. 62 (1991) 113-121.
10. VAN BRAKEL, W. H., DE SOLDENHOFF, R. and Mc-DoUGALL, A. C. The allocation of leprosy patients into paucibaeillary and multibacillary groups for multidrug therapy, taking into account the number of body areas affected by skin or nerve lesions. Lepr. Rev. 63 (1992) 231-245.
11. VAN BRAKEL, W. II., KHAWAS, I. B. and LUCAS, S. B. Reactions in leprosy: an epidemiological study of 386 in West Nepal. Lepr. Rev. 65 (1994) 190-203.
12. WHO Expert Committee on Leprosy. Fifth report. Geneva: World Health Organization, 1977. Tech. Rep. Ser. 607.
1. Ph.D.; Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
2. M.B.B.S., M.P.H., United Mission to Nepal, P.O. Box 126, Kathmandu, Nepal.
3. M.B.B.S., Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
Received for publication on 1 July 1997.
Accepted for publication in revised form on 6 October 1997.
Reprint request to Dr. Roche at the above address or fax 977-1-290538; email roche@umn.mos.com.np