- Volume 65 , Number 4
- Page: 461–4
Role of polymerase chain reaction in the diagnosis of early leprosy
ABSTRACT
For 39 patients suspected of early leprosy, skin biopsies of the lesions were done and bisected. One piece was used for histopathologic examination and the other for polymerase chain reaction (PCR) studies to detect Mycobacterium leprae. The diagnosis of early leprosy was made clinically in 14 patients and by histopathologic study in 26 patients. Acid-fast bacilli were seen in the histopathologic sections of only two patients, and M. leprae were detected using PCR techniques in 11 patients. In one patient the diagnosis of leprosy was made only because of the detection of M. leprae in the PCR study. Since even in endemic countries the profile of leprosy is changing, detection of leprosy lesions in their early stages has become increasingly important. Since the finding of M. leprae is crucial in the confirmatory diagnosis of early leprosy, it is suggested that PCR studies to detect M. leprae be done wherever possible in conjunction with histopathologic examination. It is also recommended that the feasibility and the cost-effectiveness of both of these methods to find M. leprae be evaluated.RÉSUMÉ
On a prélevé et coupé en fragments des biopsies cutanées des lésions de 39 patients présentant une lèpre débutante. Un fragment a été utilisé pour l'examen histopathologique, et l'autre pour des examens avec la réaction de polymerase en chaîne (PCR) pour détecter le Mycobacterium leprae. Le diagnostic de lèpre débutante a été établi cliniquement chez 14 patients, et par examen histopathologique chez 26 patients. Des bacilles acido-résistants ont été vus dans les coupes histopathologiques de deux patients seulement, et du M. leprae a été détecté chez. 11 patients par la technique de PCR. Chez un patient, le diagnostic de lèpre a été établi seulement à cause de la détection de At. leprae dans l'examen par PCR. Puisque même dans les pays endémiques le profil de la lèpre est changeant, la détection des lésions lépreuses dans leurs stades précoces a pris une importance croissante. Puisque la découverte de M. leprae est essentielle dans la confirma tion du diagnostic de lèpre débutante, nous suggérons que des examens de PCR soient réalisés, pour détecter du M. leprae, partout où c'est possible, en conjonction avec 1'histopathologic. Nous recommandons également que la faisabilité et le rapport coût-efficacité de ces deux méthodes de détection du M. leprae soient évalués.RESUMEN
Se tomaron biopsias de piel de las lesiones de pacientes con sospecha de lepra incipiente. Las biopsias se cortaron en 2 partes. Una parte se usó para estudios histopatológicos y la otra para buscar Mycobacterium leprae por la técnica de la reacción en cadena de la polimerasa (PCR). El diagnóstico de la lepra temprana se hizo clínicamente en 14 pacientes y por histopatología en 26 pacientes. Se observaron bacilos ácido resistentes en las secciones histopatológicas de sólo 2 pacientes. Por PCR M. leprae se detectó en 11 pacientes. En un paciente, el diagnóstico de lepra se hizo sólo por el resultado positivo de la PCR. La detección de las lesiones de la lepra en sus estadios incipientes es cada vez más importante porque aun en los países endémicos el perfil de la enfermedad está cambiando. Puesto que el hallazgo de M. leprae es crucial para el diagnóstico confirmatorio de la lepra temprana, se sugiere que en los estudios para detectar M. leprae se incluyan tanto PCR como el examen histopatológico. También se recomienda que se evalúe la practicabilidad y el costo-beneficio de ambos métodos para la detección de M. leprae.A hypopigmented patch on the skin is perhaps the most frequently noticed sign of early leprosy. A diagnosis of leprosy is made if the patch shows loss of sensations, especially to fine touch or pain, or if a skin smear from the patch is positive for acidfast bacilli (AFB). A biopsy study of the lesion will show infiltration of the dermis by chronic inflammatory cells and inflammation, and disorganization and partial destruction of the dermal nerves. One or more AFB may be found inside the dermal nerves, the arrector pili muscles or in the subepithelial region (3). AFB in lesions of early leprosy are not easily found using histopathologic techniques. In recent years polymerase chain reaction (PCR) techniques are being successfully used to demonstrate the presence of AFB in small numbers in tissue (11). Further, detection of Mycobacterium leprae by PCR techniques is specific and as few as 10 to 100 bacilli can be detected in a skin biopsy (7). In this study, we attempt to correlate the clinical and histopathological appearances of lesions diagnosed or suspected to have leprosy with the finding of M. leprae using PCR technology, and to assess whether this new test for demonstrating the presence of M. leprae will enhance our ability to diagnose early leprosy.
MATERIALS AND METHODS
The St. Thomas Hospital and Leprosy Centre at Chettupattu, India, is situated in an area endemic for leprosy in Tamil Nadu. There is a leprosy control program covering about 450,000 people and an outpatient skin clinic where patients with leprosy and other dermatologic complaints are seen regularly. From among these, 39 patients with hypopigmented skin lesions suspected to have early leprosy were chosen for the study. A thorough clinical examination of the skin and peripheral nerves was made. Skin sensation on the patch was tested and skin smears from the lesions were done for all patients. A definite diagnosis of leprosy was made when the lesion had hypopigmentation and loss of sensations (1,12). The lesion was classified as polar tuberculoid (TT) if it had a well-defined margin and a healing center. A hypopigmented skin lesion with a somewhat ill-defined margin and with definite loss of sensations was classified as borderline tuberculoid (BT). Indeterminate (I) lesions were vague and ill-defined hypopigmented patches with slightly impaired sensations (1).
A biopsy of the lesion was carried out using a 4-mm punch, and it was then divided into two parts. One half was fixed in 10% buffered neutral formalin for paraffin sections; several 5-/Um sections were made: three sections were stained with hematoxylin and eosin (H&E) and another three sections were stained for AFB using a modified Fite's stain (6). The H&E sections were examined for evidence of leprosy using an oil immersion lens. Those with few scattered periadnexal inflammatory cells with no specific pathological changes were described to have nonspecific chronic inflammation (NCI). When a lesion has well-organized granulomas with collections of epithelioid cells in the middle and a large number of lymphocytes at the periphery, it is classified as TT leprosy. A lesion with a poorly organized granuloma and fewer lymphocytes diffusely present within the granuloma, admixed with epithelioid cells, is classified as BT leprosy. AFB can be absent in both TT and BT and are more often present in BT (5,9). In indeterminate leprosy, in addition to scattered clumps of mononuclear cells around skin adnexa there is infiltration of the dermal nerves by chronic inflammatory cells followed by disorganization and partial destruction. AF B may be found inside the dermal nerves or arrector pili muscles (3).
The other portion of the skin biopsy was placed in 70 % ethyl alcohol and processed for PC R studies for M. leprae (11).
RESULTS
Of the 39 patients, 21 were males and 18 were females with ages ranging from 8 to 45 years (mean 21. 5 years). During clinical examination 37 patients had a single hypopigmented skin lesion; of the remaining 2, one had 2 skin lesions and the other had 3. The peripheral nerves did not show any significant change, and the skin smears were negative in all 39 patients. Although all of the 39 were suspected to have leprosy, a definite diagnosis on the basis of clinical examination by an experienced leprologist (JJ), using the criteria described earlier (1,12), was offered in only 14 patients who were classified as: 1 indeterminate, 4 TT and 9 BT (Table 1). The other 25 were found to have noncharacteristic hypopigmented macules (NHM).
Upon histopathological examination 26 patients were diagnosed as having leprosy and classified, using the criteria described earlier, as: 6 indeterminate, 3 TT and 17 BT. The other 13 patients were found to have only NCI (The Table). AF B were found in only 2 of 26 patients diagnosed as having leprosy. All of the 14 clinically diagnosed as having leprosy were confirmed during histopathological examination.
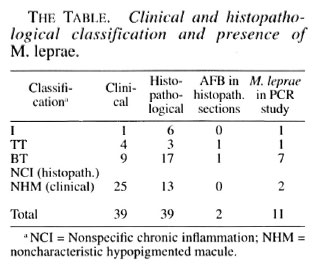
In our PC R study, evidence for the presence of M. leprae was found in 11 biopsies (The Table); of these, two need special mention. Although both patients were clinically suspected to have leprosy, the histopathological examination revealed only NCI. Upon receiving the PC R report showing evidence of M. leprae, a second biopsy from the same skin lesion was done for both patients. One showed typical histology consistent with indeterminate leprosy although no AF B were found; the other again showed only NCI. Of the other 9 PCR-positive patients, 7 were BT, 1 TT and 1 indeterminate leprosy, according to the histopathological study. Both patients, one TT and one BT who showed AF B in histopathology sections, were positive for M. leprae in PC R studies also.
DISCUSSION
In this study of 39 patients with skin lesions suspected as having early leprosy, 14 were diagnosed as leprosy clinically and 26 histopathologically. By microscopy M. leprae were found in only 2 but using PCR studies the presence of M. leprae was detected in 11 specimens. PC R increased by five to six times the frequency of finding M. leprae in tissue specimens from paucibacillary patients. This confirms the findings of other workers who have described high sensitivity and near-perfect specificity of PC R studies to detect M. leprae (2,8,10,13).
It has been reported that an increase in the number of AFB-stained sections examined will increase the frequency of detecting AF B in tuberculoid leprosy (4). Similarly, an increase in the number of PC R samples studied increased the frequency of finding M. leprae in tissue specimens (13). It is reasonable, therefore, to suggest that M. leprae are present in detectable numbers in almost all active leprosy lesions but that they are so widely dispersed in tissues that the degree of detection will depend upon the number of different samples examined. It is well known that definite histopathologic diagnosis of early lesions of leprosy depended entirely upon the finding of one or more AF B (3). Therefore, PC R study, which appears to be more sensitive than histopathological examination to detect M. leprae, could be a very good and useful adjunct in the diagnosis of early leprosy. However, we suggest that the feasibility and cost-effectiveness of these two methods to detect M. leprae be evaluated under different laboratory conditions.
With the availability of numerous effective antileprosy drugs, leprosy is being rapidly controlled throughout the world. The profile of leprosy patients presenting in an outpatient clinic is also radically changing, and more and more early lesions are encountered. Therefore, any additional tool for use in detecting early lesions will be a great advance in the field of early diagnosis. Detection of M. leprae using PCR methodology is a very new test, and has only recently emerged as a diagnostic tool. It is, at present, too elaborate and expensive to be used in routine diagnostic work. Improving the techniques of PCR study and making it cheaper is imperative. Its possible use in leprosy reference laboratories for diagnosis is unquestionable. In this connection, it is worth pointing out that one of our patients initially diagnosed as nonspecific chronic inflammation of skin histopathologically was later confirmed as indeterminate leprosy only with the assistance of the PCR results. It is suggested that PCR techniques for detecting M. leprae may profitably be used in association with histopathological studies for patients suspected of leprosy from very low-endemic areas and from nonendemic countries.
It was feared that the PCR technique is so sensitive that there may be many false-positives. In this study, oniy one specimen may be considered as false-positive because even the second biopsy from the PCR-positive lesion came up with negative histopathology.
Acknowledgment. We acknowledge with gratitude the financial support received from American Leprosy Missions International, Greenville, South Carolina, U.S.A. We are grateful to Mr. K. Rajanna and Mrs. Naoko Robbins for technical help and to Miss K. Jayanthi for secretarial assistance.
REFERENCES
1. BRYCESON, A. and PFALTZGRAFF, R. E. Leprosy. 3rd edn. New York: Churchill Livingstone, 1990, pp. 29-43, 57.
2. DE WIT, M. Y. L., FABER, W. R., KRIEG, S. R., DOUGLAS, J. T., LUCAS, S. B., ASUWAT, N. M., PAT-TYN, S. R., HUSSAIN, R., PONNIGHAUS, J. M., HARTSKLLRL, R. A. and KLASTKR, P. R. Application of PCR for detection of M. leprae in skin tissues. J. Clin. Microbiol. 29 (1991) 906-910.
3. FINE, P. E. M., JOB, C. K., LUCAS, S. B., MEYERS, W. M., PONNIGHUAS, J. M. and STERNE, J, B. C. Extent, origin and implications of observer variation in histopathological diagnosis of suspected leprosy. Int. J. Lepr. 61 (1993) 270-282.
4. FLEURY, R. F. and ARANDA, C. M. Detection of AFB in tuberculoid biopsies. (Letter) Int. J. Lepr. 63 (1995) 103.
5. JOB, C. K. and CHACKO, C. J. G. A simplified 6 group classification of leprosy. Lepr. India 54 (1982)26-32.
6. JOB, C. K. and CHACKO, C. J. G. A modification of Fite's stain for demonstration of M. leprae in tissue sections. Indian J. Lepr. 58 (1986) 17-18.
7. MISRA, N. RAMLSII, V., MISRA, R. S., NARAYAN, N. P. S., COLSTON, M. J. and NATII, I. Clinical utility of LSR/A 15 gene for M. leprae detection in leprosy tissues using PCR. Int. J. Lepr. 63 (1995) 35-41.
8. RAH, A., DONOGHUE, H. D. and STANFORD, J. L. Application of PCR for detection of M. leprae DNA in specimens from treated patients. Int. J. Lepr. 63(1995)42-45.
9. RIDLEY, D. S. and JOB, C. K. The pathology of leprosy. In: Leprosy. Hastings, R. C, ed. New York: Churchill Livingstone, 1985, p. 110.
10. SANTOS, A. R., Di; MIRANDA, A. B., SARNO, E. N., SUEFYS, P. N., and DEGRACE, W. M. Use of PCRmcdiated amplification of M. leprae DNA in different types of clinical samples for the diagnosis of leprosy. J. Med. Microbiol. 39 (1993) 298-304.
11. WILLIAMS, D. L., GILLIS, T. P., BOOTH, R. J., LOOKER, D. and WATSON, J. D. The use of specific DNA probe and PCR for detection of M. leprae. J. Infect. Dis. 162(1990) 193-200.
12. WORLD HEALTH ORGANIZATION. A guide to leprosy control. 2nd edn. Geneva: World Health Organization, 1988, p. 19.
13. YOON, K., CHAO, S., LEE, S. M., ABAI.OS, R. M., CELLONA, R. V., FAJARDO, T. T, JR., GUIDO, L. S., DELA CRUZ, E. S., WALSH, G. P. and KIM, J. Evaluation of PCR amplification of M. leprae -specific repetitive sequence in biopsy specimens from leprosy patients. J. Clin. Microbiol. 31 (1993) 895-899.
1. M.D., F.R.C.Path., Consultant Pathologist; Department of Leprosy, St. Thomas Hospital and Leprosy Center, Chettupattu 606 801, T. S. District, Tamil Nadu, India.
2. M.B.B.S., D.T.M.&H., Head, Department of Leprosy, St. Thomas Hospital and Leprosy Center, Chettupattu 606 801, T. S. District, Tamil Nadu, India.
3. Ph.D., Molecular Biologist; Molecular Biology Department, GWL Hansen's Disease Center at Louisiana Stale University, P.O. Box 25072, Baton Rouge, LA 70894, U.S.A.
4. Ph.D., Chief, Molecular Biology Department, GWL Hansen's Disease Center at Louisiana Stale University, P.O. Box 25072, Baton Rouge, LA 70894, U.S.A.
Received for publication on 4 January 1996; accepted for publication in revised form on 13 May 1996.