- Volume 65 , Number 4
- Page: 465–8
IgM anti-phenolic glycolipid-I antibody measurements f rom skin-smear sites: correlation with venous antibody levels and the bacterial index
ABSTRACT
Measurements of anti-phenolic glycolipid-I antibodies were made in 200 matched samples of capillary blood f rom the skin-smear site, venous blood collected on filter paper, and sera. A close correlation among the three samples was observed and a weaker correlation among the antibody levels and the average and skin-smear bacterial index. Capillary blood f rom the skinsmear site had a consistently higher level of antibodies in each sample than did the sera. The collection of capillary blood f rom skinsmear sites is a convenient and economical method of obtaining samples for serology and for measuring local antibody levels, and it may be more sensitive than measurements of antibodies in sera.RÉSUMÉ
Des mesures d'anticorps anti-glycolipide phénolique I ont été réalisées sur 200 échantillons appariés de sang capillaire prélevé au niveau de sites de frottis cutanés, de sang veineux récolté sur papier filtre et de scrum. On a observé une correlation étroite entre les trois échantillons, ainsi qu'une correlation plus faible entre les taux d'anticorps et l'indice bactérien moyen des frottis cutanés. Le sang capillaire prélevé au niveau des sites de frottis cutanés avait régulièrement un taux plus élevé d'anticorps au niveau de chaque échantillon que ne l'avait le serum. La récolte de sang capillaire au niveau de sites de frottis cutanés est une méthode aisée et bon marché d'obtenir des échantillons pour la sérologie et pour mesurer les taux locaux d'anticorps, et pourrait être plus sensible que la mesure du taux d'anticorps dans le serum.RESUMEN
Se midieron los niveles de anticuerpos anti-glicolípido fenólico-I en 200 muestras apareadas de sangre capilar, de sangre venosa colectada sobre papel filtro, y de suero. Se observó una estrecha correlación entre los resultados obtenidos en las 3 muestras pero sólo una débil correlación entre los niveles de anticuerpos y los índices bacterianos promedio en la linfa cutánea. En todos los casos, la sangre capilar tomada del sitio muestreado para la búsqueda de bacilos de la lepra en la linfa cutánea tuvo niveles de anticuerpos consistentemente más altos que los encontrados en sangre venosa. La colección de sangre capilar de los sitios muestreados pare bacilos en la linfa cutánea es una forma conveniente y económica de obtener pequeñas muestras de sangre para medir los niveles de anticuerpos anti-micobacterianos y los resultados pueden ser más sensibles que los encontrados al medir los anticuerpos en el suero de la sangre venosa.Measurements of IgM anti-phenolic glycolipid-I (PGL-I) antibodies in leprosy are of value for assessing the extent of the disease (4,5) and the response to therapy (6). To assess the levels of antibodies in lesions and to minimize venipunctures we have assessed the accuracy of antibody measurements made from capillary blood collected on filter paper from the skin-smear site. We have assessed whether such measurements correlate with serum antibody levels and with venous blood collected onto filter paper and investigated whether local antibody levels also reflect the bacterial load at the skin-smear site.
MATERIALS AND METHODS
Subjects. After giving informed consent, 192 consecutive patients attending Anandaban Leprosy Hospital for routine skin smears were included in the study. These were 129 males and 63 females aged between 12 and 81 years. Patients were classified according to the Ridley-Jopling classification on clinical and bacteriological criteria (3). The patient groups consisted of 7 suspect cases, 1 indeterminate case, 19 tuberculoid, 47 borderline tuberculoid, 14 borderline, 56 borderline lepromatous, 40 lepromatous and 8 primary neuritic cases. Eight patients were tested on two separate occasions; 106 patients were under treatment at the time of testing. There were four patients on paucibacillary multidrug therapy (PB-MDT) and 102 on multibacillary multidrug therapy (MB-MDT). Patients had been treated for 0 to 79 months. There maining 79 patients had completed treatment 1 to 10 years previously.
Samples. Venous blood (2 ml) was drawn from an arm vein and a single drop placed on filter paper (Whatman Scientific, Ltd., Kent, U.K.). The remaining blood was placed in a plain sterile tube and allowed to clot. Skin smears were collected from four standard sites from each patient: one each from both earlobes, the right arm and the right thigh. After collection of serous fluid for smears, a drop of capillary blood from the right earlobe was collected onto filter paper.
Assays. The slit-skin smears were stained by the Ziehl-Neelsen technique and the number of acid-fast bacilli (AFB) at each site was recorded on a logarithmic scale. An average bacterial index (BI) was calculated as the arithmetic mean of the indices at the four sites.
Filter-paper samples were allowed to dry, transported to the laboratory, and were stored for up to a month without significant loss of antibody. A 6-mm circle of bloodsoaked filter paper was punched out and soaked overnight at room temperature in 0.5 ml phosphate buffered saline containing 0.05% Tween 20 (PBST), pH 7.4. This was calculated to be equivalent to a 1 in 50 dilution of blood and used directly in the anti-PGL-I enzyme-linked immunosorbant assay (ELISA).
IgM anti-PGL-I antibodies were measured as described previously (4). Wells of a flat-bottom, 96-well ELISA tray (Dynatech, Alexandria, Virginia, U.S.A.) were coated overnight with 250 ng/ml of the glycoconjugate disaccharide-bovine serum albumin (dBSA) in 0.01 M carbonate buffer, pH 9.6. Wells were washed in PBST and blocked with 200 µ l of 1% (w/v) bovine serum albumin (BSA) in PBS for 1 hr at 37ºC; 100 µ l of sera diluted 1:300 in 10% normal goat serum (NGS) or undiluted eluate from filter-paper specimens was added to wells for 1 hr. Wells were washed four times with PBS, and 100 µ l of goat anti-human IgM-horseradish peroxidase conjugate diluted 1: 4000 in 10% NGS was added for an additional 1 hr. Plates were again washed four times before the addition of 100 µ l 0.4 g/L o-phenylenediamine (OPD; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) in 0.05 M citrate-phosphate buffer, pH 5.0, containing 0.006% hydrogen peroxide for 10 min at room temperature. The color reaction was stopped with the addition of 100 µ l 2.5 N sulfuric acid, and the plates were read at 492 nm in a Multiskan ELISA plate reader (Flow, Irvine, Scotland). Samples with an absorbanee greater than 0.199 (being the mean plus three standard deviations of samples from 91 healthy Nepali subjects) were considered positive.
Statistics. The significance of differences in the proportions seropositive in the different groups was tested with the chi-squared statistic using the Yates' correction. Differences in antibody levels detected in different sites was tested by the Wilcoxon matched pairs test. Correlations between antibody levels from different samples and antibody levels and the BI were tested by the Spearman rank correlation test.
RESULTS
Of the 200 samples tested in this study, positive antibody levels to PGL-I were found in the capillary blood from the earlobe skin-smear site in 67 samples compared with 62 sera samples and 60 samples of venous blood collected onto filter paper (Table 1); 52 patients had positive antibody levels in all three samples, 8 in two of three samples and 16 in one sample only. In total, 76 patients were positive in at least one of the three samples collected. The proportion of seropositives increased from tuberculoid to lepromatous across the leprosy spectrum. There was no significant difference in the proportion of seropositives between different samples in any leprosy class. In paucibacillary (TT/BT) patients, 10/65 (15%) were positive in sera and capillary blood and 6/65 (9%) were positive in venous blood on filter paper. In lepromatous (BL/LL), 45/96 (47%) were positive in sera, 50/96 (52%) in capillary blood samples, and 47/96 (49%) in venous blood collected on filter paper.
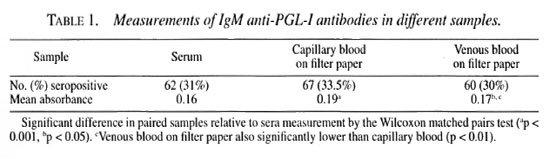
Overall, the absorbanee in the capillary blood sample was significantly higher than that in the serum sample from the same patient (Wilcoxon rank sum test; z = -4.5125, p <0.001) and that in the venous blood collected on filter paper (z = -2.8, p <0.005). Venous blood collected on filter-paper samples also gave a higher absorbanee than did sera (z = -2.4, p <0.05).
Eight patients were sampled twice in 3 months. There was an average variation in absorbanee between samples of -0.002 for sera samples, 0.023 for capillary samples, and 0.03 for venous blood samples on filter paper. Variations in individual patients were reflected in changes of similar magnitude in all three samples.
Of the 192 patients, 42 were skin-smear positive for at least one site and in 38 patients AFB were found in samples from the earlobe. There was a significantly greater proportion of seropositive patients among patients with a positive slit-skin smear as measured in any of the three samples (Table 2).
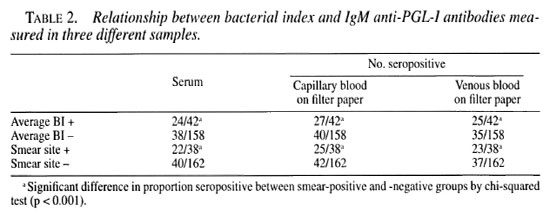
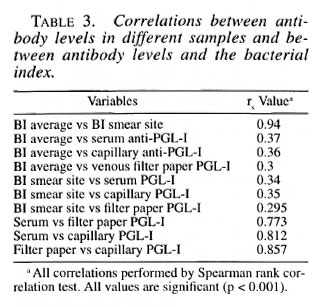
The average BI correlated strongly with the BI of the earlobe. Anti-PGL-I antibody levels, measured in any of the three samples were significantly correlated with the average and earlobe BI in individual patients. The antibody levels in patients in the three samples were also highly correlated (Table 3).
DISCUSSION
Over the past decade, measurements of anti-PGL-I antibodies have been applied in a wide variety of settings and assessed for their application to the diagnosis, classification and management of leprosy. We have detailed in previous publications a correlation of antibody levels with the extent of leprosy disease and the BI in untreated patients (4,5). In addition we have demonstrated a decline in the anti-PGL-I antibody levels in patients on multidrug therapy (MDT) of approximately 50% per year (6). This decline in antibody levels corresponded with the fall in the BI over the same period.
The detection of anti-PGL-I antibodies in dried blood samples collected on filter paper has been described by other workers (2). In these studies, as well as our own unpublished data, antibody levels in dried blood correlate well with serum antibody levels. We have used this method to extend the measurements of PGL-I antibodies to the capillary blood from skin-smear sites in order to evaluate the significance of PGL-I antibodies at the site of infection. While the capillary blood antibody levels correlated well with levels in the serum, antibody levels were slightly but significantly higher in most patients' capillary blood compared with their serum samples. This may reflect a higher concentration of PGL-I antibodies at the site of infection compared with circulating antibody levels in the venous blood.
The measurement of the BI remains a fundamental part of the diagnosis of leprosy, and in most programs a measure of the success of leprosy chemotherapy. The earlobe is a commonly sampled site, often remaining positive after other sites and patches have become negative. This is reflected in our finding that the capillary blood samples from the earlobe were also positive in a greater number of patients than in sera.
Measuring capillary blood from suspect skin lesions may be a useful additional measure in concluding that a lesion is due to leprosy. Monitoring the local levels of PGL-I antibodies in the lesion may be of use in the monitoring of type 2 reactions in which circulating antibody levels have been shown to fluctuate (1). Furthermore, the changes in PGL-I antibodies in the lesion reflect the success of chemotherapy, and would be a useful parameter in addition to the BI in assessing the response to treatment.
We suggest that capillary blood from skin-smear sites is an easy and noninvasive method of collecting serological samples. The collection of samples onto filter paper would allow peripheral health posts to send samples to a central laboratory. Such samples could provide useful measures to field programs in the management of leprosy.
Acknowledgment. We would like to thank the patients and staff of the Anandaban Hospital for their kind cooperation without which this study would not have been possible. Anandaban Hospital and the Mycobacterial Research Laboratory are fully supported by The Leprosy Mission International. Wc thank the IM-MYC program of the World Health Organization for the provision of the disaccharide BSA antigen.
REFERENCES
1. ANDREOLI, A., BRETT, S., DRAPER, P., PAYNE, S. N. and ROOK, G. A. W. Changes in circulating antibody levels to the major phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int. J. Lepr. 53(1985)211-217.
2. CHANTEAU, S., PI.ICHART, R., BOUTIN, J.-P., Roux, J. and CARTEL, J.-L. Finger-prick blood collection and computer assisted enzyme-linked immunosorbent assay for large scale serological studies on leprosy. Trans. R. Soc. Trop. Med. Hyg. 83 (1989) 414-416.
3. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34 (1966) 255-273.
4. ROCHE, P. W., BRITTON, W. J., FAILISUS, S. S., LUD-WIG, H., THEUVENET, W. J. and ADIGA, R. B. Heterogeneity of serological responses in paucibacillary leprosy: differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int. J. Lepr. 58 (1990) 319-327.
5. ROCHE, P. W., BRITTON, W. J., FAILISUS, S. S., WILLIAMS, D., PRADHAN, H. M. and THEUVENET, W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58 (1990) 480-490.
6. ROCHE, P. W., FAILBUS, S. S., NEUPANE, K. D., THEUVENET, W. J. and BRITTON, W. J. Serological monitoring of the response to chemotherapy in leprosy patients. Int. J. Lepr. 61 (1993) 35-43.
1. M.B.B.Ch.; Anandaban Leprosy Hospital, P.O. Box 151, Kath-mandu, Nepal.
2. M.B.B.S.; Anandaban Leprosy Hospital, P.O. Box 151, Kath-mandu, Nepal.
3. Anandaban Leprosy Hospital, P.O. Box 151, Kath-mandu, Nepal.
4. M.Sc; Anandaban Leprosy Hospital, P.O. Box 151, Kath-mandu, Nepal.
5. Ph.D., Anandaban Leprosy Hospital, P.O. Box 151, Kath-mandu, Nepal.
Received for publication on 17 March 1997.
Accepted for publication in revised form on 9 July 1997.
Reprint requests to Dr. Roche at the above address or fax 977-1-290-538.