- Volume 65 , Number 4
- Page: 477–86
Epitope mapping of twelve monoclonal antibodies against the phenolic glycolipid-I of M. leprae
ABSTRACT
Epitope mapping of 12 monoclonal antibodies (MAbs) directed to the trisaccharide part of the phenolic glycolipid-I (PGL-I) of Mycobacterium leprae was carried out by using the set of chemically synthesized sugar-BSA conjugates. The results can be summarized as follows: mAb (1-21), mAb (1-24) and mAb (1-25) recognized the outer (nonreducing end) monosaccharide of the trisaccharide chain of PGL-I. However, the affinity of these MAbs to the outer monosaccharide was weak. They required the contributions of some parts of the second sugar for enough affinity. MAbs ml 6A12, ml 8A2, ml 8B2, and PG2 B8F recognized the outer disaccharide. MAb F47-21-3 recognized the outer disaccharide and some parts of the third sugar. MAb SF 1 recognized the trisaccharide of PGL-I. MAb 3D1-A9 recognized the phenol group and the structure around the branching point on the carrier protein in addition to the trisaccharide. MAbs DZ 1 and 2G3-A8 had unique characters which recognized the inner part of the sugar chain. MAb DZ 1 recognized the inner (reducing end) disaccharide. MAb 2G3-A8 recognized the inner monosaccharide, phenol group and the structure around the branching point on the carrier protein. All of the MAbs tested, except for ml 6A12, recognized the anomeric configurations in the sugar parts they recognized; ml 6A12 recognized the anomeric configuration only within the outer disaccharide. This set of MAbs, which were well defined on their binding specificity, promises to be an effective tool for the immunological study of PGL-I and the clinical assessment of leprosy.RÉSUMÉ
La carte des epitopes de 12 anticorps monoclonaux (Acm) dirigés contre la fraction trisaccharidique du glycolipide phénolique I (PGL-I) de Mycobacterium leprae a été réalisée à l'aide de la série de conjugués sucre-BSA synthétisés chimiquement. Les résultats peuvent être résumés comme suit : les Acm (1-21), Acm (1-24) et Acm (1-25) reconnaissaient le monosaccharide externe (extrémité non-réductrice) de la chaîne trisaccharidique de PGL-I. Cependant, l'affinité de ces Acm pour le monosaccharide terminal était faible. La contribution de certains fragments du deuxième sucre était nécessaire pour que l'affinité soit suffisante. Les Acm ml 6A12, ml 8A2, ml 8B2 et PG2 B8F reconnaissaient le disaccharide terminal. L'Acm F47-21-3 reconnaissait le disaccharide terminal et certaines portions du troisième sucre. L'Acm SF1 reconnaissait le trisaccharide de PGL-I. L'Acm 3D1-A9 reconnaissait le groupe phenol et la structure autour du point de branchement sur la protéine porteuse, en plus du trisaccharide. Les Acm DZ1 et 2G3-A8 avaient des caractères uniques qui reconnaissaient la partie interne de la chaîne de sucre. L'Acm DZ1 reconnaissait le disaccharide interne (extrémité réductrice). L'Acm 2G3-A8 reconnaissait le monosaccharide interne, le groupe phenol et la structure autour du point de branchement sur la protéine porteuse. Tous les Acm testés, à l'exception du ml 6A12, reconnaissaient les configurations anomériques dans les parties des sucres qu'ils reconnaissaient; ml 6A12 reconnaissait la configuration anomérique seulement dans le disaccharide externe. Cette série d'Acm, qui ont été bien définis quant à leurs spécificités de liaisons, promet d'être un outil efficace pour l 'étude immunologique du PGL-I et l'évaluation clinique de la lèpre.RESUMEN
Se analizó la reactividad de 12 anticuerpos nionoclonales (mAbs) dirigidos contra cl trisacárido del glicolípido fcnólico-I (PGL-I) de Mycobacterium leprae usando una serie de azúcares sintéticos conjugados a BSA. Los resultados pueden resumirse como sigue: los mAbs (1-21), (1-24) y (1-25) reconocieron al monosacárido externo (extremo no reductor) del trisacárido. Sin embargo, la afinidad de estos mAbs por el monosacárido fue débil y se requirió de la contribución de algunas partes del segundo azúcar para mayor afinidad. Los mAbs ml 6A12, ml 8B2 y PG2 B8F, reconocieron al disacárido externo. El mAb F47-21-3 reconoció al disacárido externo y algunas partes del tercer azúcar. El mAb SF1 reconoció al trisacárido del PGL-I. El mAb 3D1-A9 reconoció al grupo fenol y la estructura alrededor del punto de ramificación de la proterna acarreadora, además del trisacárido. Los mAbs DZ1 y 2G3-A8 reconocieron la parte interna de la cadena del trisacárido. El mAb DZ1 reconoció al disacárido interno (extremo reductor). El mAb 2G3A8 reconoció al monosacárido interno, al grupo fenol, y a la estructura alrededor del punto de ramificación de la proteína acarreadora. Todos los anticuerpos monoclonales probados, excepto el ml 6A12, reconocieron las configuraciones anoméricas de los azúcares reconocidos; el ml 6A12 reconoció la configuración anomérica sólo dentro del disacárido externo. Esta serie de anticuerpos monoclonalcs, los cuales fueron bien definidos en cuanto a su especificidad de enlazamiento, promete ser de gran utilidad en el estudio inmunológico del PGL-I y en el establecimiento clínico de la enfermedad.Monoclonal antibodies (MAbs) because of their unique specificity and high potential affinity for specific epitopes, which may be targets on clinically important molecules, deserve close and accurate examination as to their binding specificities. The use of MAbs for identification of the location of specific antigens in tissues and their presence in clinical samples is one of their very important potential applications. The understanding of a disease such as leprosy, caused by Mycobacterium leprae which has to date resisted all attempts at in vitro cultivation, can be greatly enhanced by the application of MAbs.
To this end, a number of MAbs have been produced against antigens of M. leprae. Among these MAbs, MAbs directed to the phenolic glycolipid-I (PGL-I) of M. leprae were one of the major groups. PGL-I is of interest because of the high specificity to leprosy and the immunological roles of the molecule in the host (7,15,18). Recently, polyclonal antibodies and MAbs have been used for the direct detection of M. leprae or the PGL-I antigen in tissue of clinical specimens such as serum and urine (2,6,10) . However, little is known about the binding sites of MAbs reacting with the sugar determinants of PGL-I. The antigenic determinant of PGL-I is shown to be the trisaccharide, 0-(3,6-di-(0-methyl- β -D-glucopyranosyl)(1→4)-0-(2,3-di-O-methyl-α-L-rhamnopyanosyl)-( 1→2)-(3-0-methyl-α-L-rhamnopyranose), which can be synthesized by chemical methods (3). With the development of the methods of synthesizing the antigenic structures for these sugars, it is now possible to obtain a variety of sugars with the closely related structures in the form of a conjugate with bovine serum albumin (BSA) (1,3,4,5). The combination of these conjugates, which are well defined on the chemical structures, and a variety of MAbs provides an excellent model for studying the mechanism by which antibodies recognize the sugar epitopes.
In this paper, we characterize the binding site specificity of MAbs produced by different mouse hybridoma cell lines for the sugar epitopes of PGL-I. The MAbs were investigated for the participation of individual sugars in their binding sites by examining the reactivity in ELISA and in ELISA inhibition assays with the synthetic constructs mimicking the mono-, di- and trisaccharide of the PGL-I molecule and the trisaccharides with different anomeric configurations.
MATERIALS AND METHODS
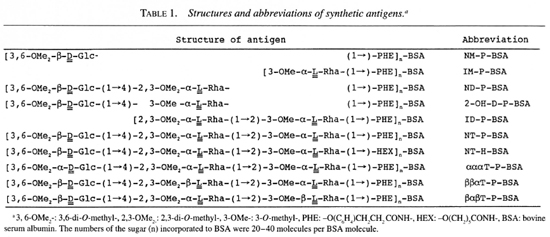
Antigens. Ten sugars (Table 1) closely related to the trisaccharide of PGL-I were synthesized by the methods reported previously or by similar methods (3,4). They were coupled to bovine serum albumin (BSA, Fraction V; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) by the acyl azide method as described by Lemieux, et al (8). Synthesized sugar-BSA conjugates (synthetic antigens) contained 20-40 moles of the sugar molecule per one mole of BSA. The synthetic antigen NT-P-BSA had the same structure as the trisaccharide and phenol group of PGL-I. NT-P-BSA was used as the control for the reactivity of MAbs. The sugar structure of NT-H-BSA was the same as that of PGL-I but it did not have a phenol group.
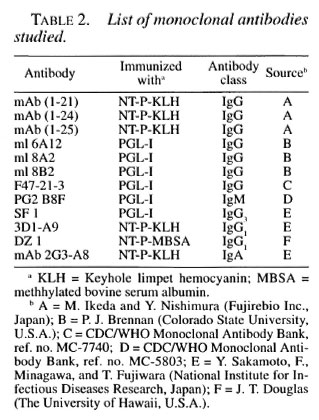
Monoclonal antibodies. Twelve MAbs directed to the sugar part of PGL-I were tested in this study. The sources and characteristics of these MAbs are listed in Table 2.
Buffers. Carbonate buffer (pH 9.3) consisting of 35 mM sodium hydrogen carbonate and 15 mM sodium carbonate was used for the coating of the antigen. The washing buffer and dilution buffer have been previously reported(4).
ELISA. ELISA was conducted by the conventional method previously reported (4). Fifty pi of antigen solution in carbonate buffer containing 31.4-0.0314 nM of sugar was used. Peroxidase-conjugated rabbit immunoglobulin to mouse immunoglobulin was employed as a second antibody in the dilution of 2000-fold. Serological reactivity was expressed by A492-A660. The reactivity of MAb was measured under the dilutions of 800-1500-fold for mAb (1-21), mAb (1-24), mAb (1-25), ml 6A12, ml 8A2, ml 8B2, F47-21-3, PG2 B8F, SF 1, 3D1-A9, 2G3-A8 or 80,000-fold for DZ1, which gave the A492-A660 value of about 1.0.
ELISA inhibition assay. A microplate was coated with 50 µl of NT-P-BSA (0.314 nM as the sugar in carbonate buffer). After washing and blocking, the mixture of MAb and inhibiting antigen was added (final concentration of 31.4 nM). The plate was incubated for 1 hr at 37ºC, washed, and the remaining activity was measured by the usual ELISA method (4).
RESULTS
In order to determine the regions of the trisaccharide of PGL-I which the MAbs recognized, reactivities to various synthetic antigens were titrated against the concentrations of the synthetic antigens in an ELISA. The results were also confirmed by an ELISA inhibition assay. In order to simplify the description, three glycosidic linkages in the trisaccharide of PGL-I are described as L1, L2, and L3 from the outer end (nonreducing end) to inner end (reducing end) of the trisaccharide of PGL-I.
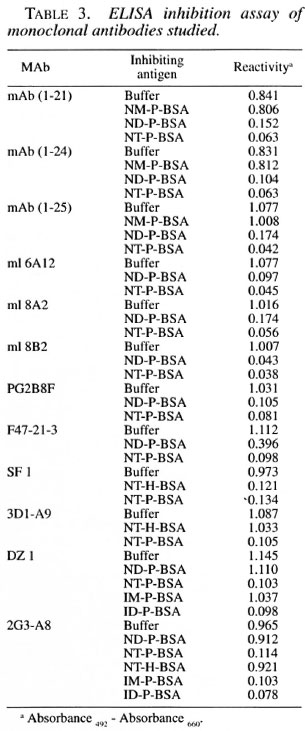
MAb (1-21). MAb (1-21) had almost the same levels of the reactivity to NM-P-, ND-P- and NT-H-BSA as that to NT-P-BSA, which had the same trisaccharide and phenol group structures of PGL-I (Fig. 1a). MAb (1-21) did not have any reactivity to IM-P- and ID-P-BSA, which lacked an outer terminal 3,6-di-O-methylglucose (NM). Thus, mAb (1-21) recognized only the outer terminal monosaccharide. In an ELISA inhibition assay, the reactivity to NT-P-BSA was not inhibited by NM-P-BSA (Table 3), although the reactivity of mAb (1-21) to NM-P-BSA was inhibited by NM-P-, ND-P-, and NT-P-BSA (data not shown). This indicates that the affinity of mAb (1-21) to NM-P-BSA was weak and that some contributions of the second sugar were required to have strong affinity to the antigen, although the specificity of the binding was determined by NM.
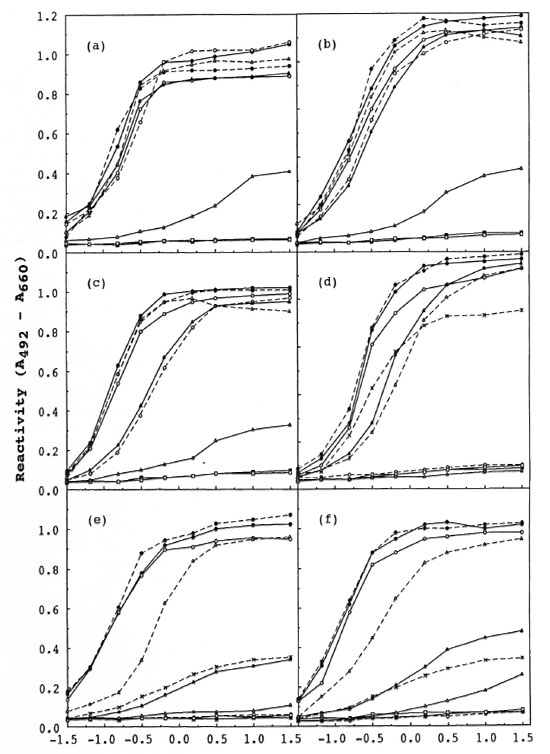
Fig. 1. Reaction kinetics of MAbs to synthetic antigens. Tested MAbs are: mAb (1-21) (a), mAb (1-24) (h),mAb (1-25) (c), ml 6Al2 (d), ml 8A2 (e) and ml 8B2 (f). Tested antigens arc: NM-P-BSA ( ), ND-P-BSA(
), ND-P-BSA( ), NT-P-BSA (
), NT-P-BSA ( ), NT-H-BSA (
), NT-H-BSA ( ), IM-P-BSA (
), IM-P-BSA ( ), ID-P-BSA (N-A), αααT-P-BSA(
), ID-P-BSA (N-A), αααT-P-BSA( ), βααTP-BSA (
), βααTP-BSA ( ), βαβT-P-BSA (
), βαβT-P-BSA ( ), and 2-OH-D-P-BSA (x---x). Reactivity to 2-OH-D-P-BSAwas not tested for mAb (1-21), mAb (1-24), or mAb (1-25).
), and 2-OH-D-P-BSA (x---x). Reactivity to 2-OH-D-P-BSAwas not tested for mAb (1-21), mAb (1-24), or mAb (1-25).
To examine whether or not mAb (1-21) recognized the anomeric configurations of three linkages (L1, L2, and L3) in the trisaccharide, an ELISA was conducted on the synthetic antigens with modified anomeric configurations (Fig. la). Modification of the anomeric configuration of L1 (αααT-P-BSA) caused a great loss of reactivity of mAb (1-21). No decrease of the reactivity was detected to the conjugates having the modified anomeric configuration of L2 (ββαT-P-BSA) or L3 (βαβT-P-BSA). Thus, mAb (1-21) recognized only the anomeric configuration of L1.
MAb (1-24). MAb (1 -24) was reactive with NM-P-, ND-P-, NT-H- and NT-P-BSA in an ELISA (Fig. lb). MAb (1-24) was inactive with ID-P- and IM-P-BSA. These results were almost the same as the profile of the recognition of mAb (1-21). In an ELISA inhibition assay, the same results with mAb (1-21) were obtained (Table 3). Thus, mAb (1-24) recognized NM, but the affinity to NM-P-BSA was weak, similar to mAb (1-21).
MAb (1-24) had the same levels of the reactivity to both ββαT-P-BSA and βαβT-P-BSA as that to NT-P-BSA in an ELISA. But only weak reactivity to αααT-P-BSA at high antigen concentration was detected, demonstrating that mAb (1-24) recognized the anomeric configuration of L1 (Fig. 1b).
MAb (1-25). MAb (1 -25) gave a similar spectrum of reactivity to that of mAb (1-21) except to NM-P-BSA (Fig. 1c). MAb (1-25) did not have any reactivity to ID-Pand IM-P-BSA. It had almost the same level of reactivity to ND-P- and NT-H-BSA as that to NT-P-BSA. The reactivity of mAb (1-25) to NM-P-BSA was different from mAb (1-21) and mAb (1-24), low at low antigen concentrations, but it had almost the same level of reactivity to NT-P-BSA at antigen concentrations higher than 1.57 nM. This result suggested that mAb (1-25) recognized NM essentially and that some parts of the central 2,3-di-6>-methylrhamnose (CM) contributed to the reactivity. In an ELISA inhibition assay, the reactivity to NT-P-BSA was not inhibited by NM-P-BSA (Table 3), although the reactivity of mAb (1-25) to NM-P-BSA was completely inhibited by NM-P-, ND-P-, and NT-P-BSA (data not shown) as was the case for mAb (1-21).
A large degree of decreasing reactivity due to the change of anomeric configuration of L1 was detected in the case of αααT-P-BSA in an ELISA. It had reduced reactivity to ββαT-P-BSA at low antigen concentration. But the decrease of the reactivity was at almost the same level as that to the monosaccharide antigen NM-P-BSA (Fig. lc). No decrease of the reactivity to βαβT-P-BSA was detected. Thus, mAb (1-25) recognized the anomeric configuration of L1.
ml 6A12. MAb ml 6A12 did not react with IM-P- and ID-P-BSA in ELISA (Fig. 1d). The reactivities to ND-P-, 2-OH-D-P-, and NT-H-BSA were close to that of NT-P-BSA. Reactivity to NM-P-BSA was not detected. In an ELISA inhibition assay the activity to NT-P-BSA was completely inhibited by ND-P- and NT-P-BSA (Table 3). Therefore, ml 6A12 recognized the outer disaccharide (ND).
In an ELISA ml 6A12 did not have any reactivity with αααT-P-BSA. It was active with ββαT-P-BSA and βαβT-P-BSA, although some decrease of reactivity was detected at a low antigen concentration, demonstrating that ml 6A12 recognized the anomeric configuration of L1 but did not recognize those of L2 and L3 (Fig. 1d). The fact that ml 6A12 was reactive with 2-OH-D-P-BSA with about 75%-85% of the reactivity seen with NT-P-BSA in an ELISA at a high antigen concentration suggests that essentially ml 6A12 did not recognize the 2-O-methyl group of CM.
ml 8A2. MAb ml 8A2 had a similar profile of reactivity to that of ml 6A12 except that ml 8A2 gave only weak reactivities to both 2-OH-D-P- and ββαT-P-BSA (Fig. 1e) in an ELISA. In an ELISA inhibition assay the reactivity to NT-P-BSA was inhibited completely by ND-P-BSA or NT-P-BSA (Table 3). Thus, ml 8A2 recognized the ND, the anomeric configurations of L1 and L2, and the 2-0-methyl group of CM.
ml 8B2. Ml 8B2 had a profile very similar to the recognition of ml 8A2 in an ELISA (Fig. 1f), leading to the conclusion that ml 8B2 recognized the ND and the anomeric configurations of L1 and L2, and the 2-0-methyl group of CM. The results of an ELISA inhibition assay supported the conclusion (Table 3).
PG2 B8F. PG2 B8F had almost the same levels of reactivity to ND-P- and NT-H-BSA as that to NT-P-BSA. It did not have the reactivity to NM-P-, IM-P-, and ID-P-BSA. PG2 B8F showed slightly reduced reactivity to 2-OH-D-P-BSA (Fig. 2a). These results showed that PG2 B8F recognized the ND and did not recognize the 2-O-methyl group of CM. An ELISA inhibition assay supported the conclusion (Table 3).
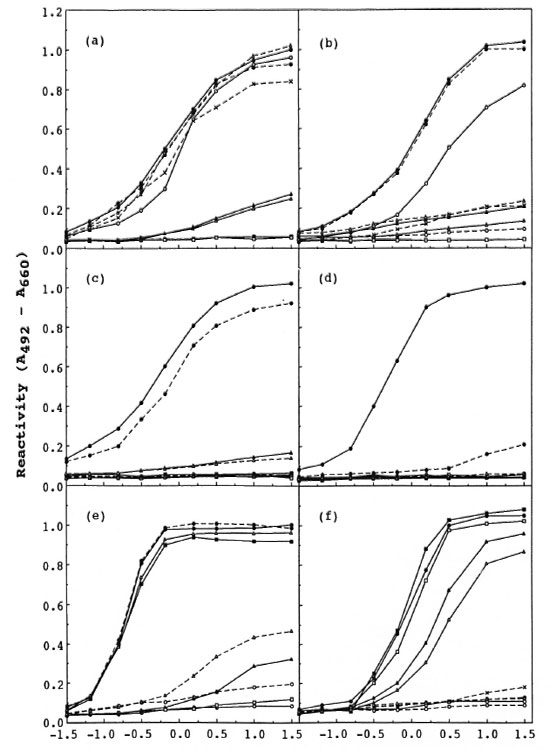
Fig. 2. Reaction kinetics of MAbs to synthetic antigens. Tested MAbs are: PG2 B8F (a), F47-21-3 (b), SF 1(c), 3D1-A9 (d), DZ 1 (c), and 2G3-A8 (f). Symbols for synthetic antigens are same as those in Fig. I. Reactivity to 2-01-1-D-P-BSA was not tested for SF 1, 3D1-A9, DZ 1, or 2G3-A8.
PG2 B8F was reactive with βαβT-P-BSA. But only slight reactivity to αααT-P and ββαT-P-BSA was found in the ELISA, showing that PG2 B8F recognized the anomeric configurations of both L1 and L2.
F47-21-3. F47-21 -3 had the same level of reactivity to NT-H-BSA as that to NT-P-BSA (Fig. 2b). Reduced reactivity to ND-P-BSA was detected. No reactivity to NM-P, 2-OH-D-P-, αααT-P-, ββαT-P-, and βαβT-P-BSA was detected. In an ELISA inhibition assay, the reactivity to NT-P-BSA was inhibited completely by NT-P-BSA, and about 62% by ND-P-BSA (Table 3). The reactivity to ND-P-BSA was inhibited by ND-P-BSA and NT-P-BSA almost completely (data not shown). Therefore, it was concluded that F47-21-3 recognized the ND, the 2-O-methyl group of CM and some part of the IM and the anomeric configurations of L1 and L2. It was found that the change of the anomeric configuration of L3 caused a complete loss of reactivity, indicating that the anomeric configuration of L3 had a considerable contribution to the recognition of F47-21-3, although the contribution of the inner disaccharide (IM) was only small.
SF 1. SF 1 had a very strict structural requirement for the expression of reactivity. SF 1 had reactivity only to NT-H-BSA other than NT-P-BSA (Fig. 2c). It was clear that SF 1 recognized the whole structure of the trisaccharide and all of three anomeric configurations. The reactivity of SF 1 to NT-P-BSA was inhibited only by NT-P-BSA or NT-H-BSA in an ELISA inhibition assay (Table 3), supporting the conclusion.
3D1-A9. 3D1-A9 had a more strict structural requirement for its reactivity than SF 1. MAb 3D1-A9 was reactive only with NT-P-BSA (Fig. 2d). MAb 3D1-A9 did not show any reactivity to NT-H-BSA. Reactivity to NT-P-BSA was inhibited by only NT-P-BSA in an ELISA inhibition assay (Table 3). MAb 3D1-A9 was inactive to PGL-I itself. Therefore, 3D1-A9 recognized the phenol group and the structure around the peptide bond at the branching point of the synthetic trisaccharide from the carrier protein in addition to the whole trisaccharide structure and all three of the anomeric configurations.
DZ 1. Differing from other MAbs tested, DZ 1 had high reactivity to ID-P-BSA and NT-P-BSA but did not have any reactivity to NM-P-, ND-P- or IM-P-BSA (Fig. 2e). DZ 1 reacted with NT-H-BSA at the same level of reactivity as to NT-P-BSA. In an ELISA inhibition assay the reactivity of DZ 1 to NT-P-BSA was inhibited by ID-P- and NT-P-BSA (Table 3), but it was not inhibited by ND-P-BSA and IM-P-BSA. The reactivity to ID-P-BSA was inhibited by ID-P- and NT-P-BSA completely (data not shown). These results showed that DZ 1 recognized only the inner disacharide (ID).
DZ 1 had the same level of the reactivity to αααT-P-BSA as to NT-P-BSA in an ELISA. DZ 1 showed only weak reactivities to both ββαT-P-BSA and βαβT-P-BSA even at high antigen concentrations, showing that DZ 1 recognized the anomeric configurations of L2 and L3.
2G3-A8. 2G3-A8 had almost the same level of reactivity to IM-P-, ID-P-BSA as to NT-P-BSA. No reactivity to NM-P-, ND-Por 2-OH-D-P-BSA was detected (Fig. 2f). In an ELISA inhibition assay, the reactivity to NT-P-BSA was inhibited by ony one of IM-P-, ID-P- and NT-P-BSA. This reactivity was not inhibited by NM-P- or ND-P-BSA (Table 3). Thus, 2G3-A8 recognized the IM. Different from DZ 1, 2G3-A8 did not have any reactivity to NT-H-BSA and its reactivity to NT-P-BSA was not inhibited by NT-H-BSA (Table 3). MAb 2G3-A8 did not have reactivity to PGL-I as was the case for 3D 1-A9. Therefore, it is concluded that 2G3-A8 recognized the IM, the phenol group, and the structure around the branching point on the carrier protein.
MAb 2G3-A8 gave a reduced level of reactivity to αααT-P- and ββαT-P-BSA compared to NT-P-BSA, but the decrease of the reactivity was small. No reactivity was detected to βαβT-P-BSA. Thus, 2G3-A8 recognized only the anomeric configuration of L3.
DISCUSSION
A detailed analysis of the recognition sites of 12 MAbs by using synthetic sugars closely related to the trisaccharide of PGL-I indicates that MAbs recognized the sugar structure of PGL-I strictly. They recognized each monosaccharide residue which consists of the trisaccharide of PGL-I and their anomeric configurations. They were classified roughly into five groups which recognized: 1) the outer monosaccharide (NM) [mAb (1-21), mAb (1-24), mAb (1-25)]; 2) the outer disaccharide (ND) (ml 6A12, ml 8A2, ml 8B2, PG2 B8F, F47-21-3; 3) the trisaccharide (NT) (SF-1) or NT, the phenol group, and the structure around the branching point on the carrier protein (3D1-A9); 4) the inner disaccharide (ID) (DZ 1); or 5) the inner monosaccharide (IM), the phenol group, and the structure around the branching point on the carrier protein (2G3-A8) as summarized in Fig. 3. Among NDrecognizing MAbs, ml 6A12, and PG2 B8F did not recognize the 2-0-methyl group on CM, indicating that the minimum regions which were recognized by these MAbs are not whole structures of the disaccharide. In cases of MAbs classified as NM-recognizing MAbs, their affinities to the NM were weak. They required the contribution of the second sugar for strong affinity. This probably means that these MAbs recognized some small part of the CM, but they are reactive even in the condition in which they bound to the antigen loosely. This was found also in F47-21-3, which had reduced activity to ND-P-BSA but was still reactive. Therefore, the grouping by the sugar residue unit is a rough one and has some ambiguity for representing the epitopes recognized by these MAbs.
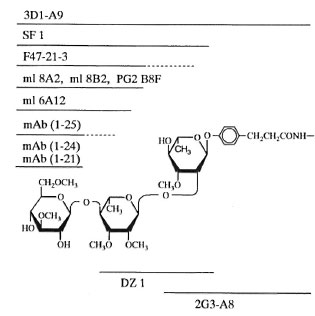
Fig. 3. Recognition sites of the MAbs. - = Regions recognized by the MAbs; = regions whichhave some contributions to the reactivity of MAbs.
It was found in this study that mice mainly produced ND-recognizing MAbs and some NM-recognizing MAbs by immunization with PGL-I or with synthetic trisaccharideprotein conjugates. A similar result was obtained for the sera of leprosy patients (12). These results are consistent with the wellknown facts in various biologically important sugar antigens, such as gangliosides and lipopolysaccharides, that anti-sugar MAbs recognize their epitopes present at the outside of the sugar chain (9,14). This explains well the earlier findings that 3,6-diO-methylglucose of PGL-I was necessary for the expression of reactivity of PGL-I to the patients' sera and that the outer disaccharide-BSA and the trisaccharide-BSA conjugates had close reactivity and specificity with human leprosy sera (1)
MAbs recognizing the inner end (reducing end) sugar (DZ 1, 2G3-A8) were also obtained, although the production of MAbs recognizing the inner part of the sugar chain is rare in most cases. One of the reasons for this may be that the conformation of NT-P in aqueous solution was the one in which the CM and IM residues were exposed to the outside. Molecular modeling by molecular force field calculation using Macro-Model programs and a nuclear magnetic resonance (NMR) study suggested that the
NT-P molecule was in the conformation in which the sugar chain is bent between the CM and IM residues of the trisaccharide chain. In this conformation, the area around C3-C4-C5 of the reducing-end IM residue and the area around C3-C4-C5 of the CM residue had a large open space to the outside, thus supporting this possibility.
This set of MAbs whose specificities were well defined provides a useful tool for many kinds of studies, such as the identification of M. leprae, the detection of PGL-I in biological specimens by using two MAbs which recognize different sites (13), purification of the synthetic saccharide antigen by an affinity chromatography, and immunohistological staining of M. leprae, etc. (6). The set of a variety of MAbs and synthetic antigens may be useful for the analysis of the composition of the polyclonal antibodies against PGL-I in patients' sera, which may have a close relationship to the health status of the patients. Among MAbs tested SF 1 required the complete structure of the whole trisaccharide of PGL-I for its reactivity, showing that SF 1 has an extremely high specificity to PGL-I. Therefore, it is especially useful for these studies.
Acknowledgment. This study was supported by a grant (06240105) from the Ministry of Education, Japan, and the Office of Research Administration, The University of Hawaii, Honolulu, Hawaii, U.S.A. The authors wish to express their sincere thanks to Drs. M. Ikeda and Y. Nishimura (Fujirebio Inc., Japan), P. J. Brennan (Colorado State University, U.S.A.), L. Zhong (University of California at Los Angeles, U.S.A.) and the IMMLEP Monoclonal Antibody Bank for providing monoclonal antibodies and The University of Hawaii monoclonal faculty for their assistance.
REFERENCES
1. CHATTERJEE, D., CHO, S.-N., STEWART, C , DOUGLAS, J. T, FUJIWARA, T. and BRENNAN, P. J. Synthesis and immunoactivity of neoglyco-proteins containing the trisaccharide unit of phenolic glycolipid-I of Mycobacterium leprae. Carbohydr. Res. 183(1988)241-260.
2. CHO, S.-N., HUNTER, S. W., GELBER, R. H., REA,T. H. and BRENNAN, P. J. Quantitation of phcnloic glycolipid of M. leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 150 (1986)560-569.
3. FUJIWARA, T., HUNTER, S. W., CHO, S.-N., As-PINALL, G. O. and BRENNAN, P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from leprosy bacillus and preparation of a disaccharide-protein conjugate for serodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
4. FUJIWARA, T. and IZUMl, S. Synthesis of the neoglycoconjugates of phenolic glycolipid-related trisaccharides for the serodiagnosis of leprosy. Argric. Biol. Chem. 51 (1987) 2539-2547.
5. GlGG, J., GIGG, R., PAYNE, S. and CONANT, R. Synthesis of propyl 4-0-(3,6-di-0-methyl-β-D-glucopyranosyl)-2,3-di-0-methyl-α-L-rhamnopyranoside. Charbohydr. Res. 141 (1985) 91-97.
6. GOTO, M., MINAUCHI, Y, NOBUHARA, Y and SATO, E. Immuno-histochemical demonstration of Mycobacterium leprae in the nervous system of longterm cured leprosy patients using a M. leprae-spe cific anti-PGL antibody. Jpn. J. Trop. Med. Hyg. 21 (1993)117-121.
7. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 25 7 (1983) 15072-15078.
8. LEMIEUX, R. U., BUNDLE, D. R. and BAKER, D. A. The properties of a "synthetic" antigen related to the human blood-group Lewis a. J. Am. Chem. Soc. 97(1975)4076-4083.
9. LUK, J. M. C, LIND, S. M., TSANG, R. S. W. and LtNDBERG, A. A. Epitope mapping of four monoclonal antibodies recognizing the hexose core domain of Salmonella lipopolysaccharide. J. Biol. Chem. 266 (1991) 23215-23225.
10. MAHON, A. C, KURLIGN, A., KEBEDE, B., BECX-BLEUMINK, M. and LEFFORD, M. J. Urinary phenolic glycolipid I in the diagnosis and management of leprosy. J. Infect. Dis. 163 (1991) 653-656.
11. MEHRA, V., BRENNAN, P. J., RADA, E., CONVIT, J. and BLOOM, B. R. Lymphocyte suppression in Leprosy induced by unique M. leprae glycolipid. Nature 308 (1984) 194-196.
12. MINAGAWA, F. Serodiagnosis of leprosy. In: Latent Infection of Bacteria and Host Response , Vol. 2. Kurata, T, and Amano, F., eds. Toyko: Saikon Publishing Co., 1996, pp. 45-55.
13. PATIL, S. A., GIRDHAR, B. K., SINGH, K. P. and SENGUPTA, U. Detection of Mycobacterium leprae antigens in sera of leprosy patients by sandwich immunoradionietric assay using monoclonal antibodies. J. Clin. Microbiol. 28 (1990) 2792-2796.
14. TAI, T., PAULSON, J. C, CAHAN, L. D. and [RIE, F. Ganglioside GM2 as a human tumor antigen (OFA-1-1). Proc. Natl. Acad. Sci. U.S.A. 80 (1983)5392-5396.
15. VACHULA, M., HOLZER, T. J. and ANDERSEN, B. R. Suppression of monocyte oxidative response by phenolic glycolipid I of Mycobacterium leprae. J. Immunol. 142 (1989) 1696-1701.
1. Ph.D., Institute for Natural Science, Nara University, 1500 Misasagi-cho, Nara 631, Japan.
2. D.V.M.; National Institute of Infectious Diseases. Leprosy Research Center, 4-2-1 Aoba-cho, Higashimurayama 189, Japan.
3. Ph.D. (deceased 2 February 1995), National Institute of Infectious Diseases. Leprosy Research Center, 4-2-1 Aoba-cho, Higashimurayama 189, Japan.
4. D.V.M., Department of Microbiology, The University of Hawaii, Honolulu, Hawaii 96822, U.S.A.
Received for publication on 24 February 1997.
Accepted for publication in revised form on 14 July 1997.