- Volume 65 , Number 4
- Page: 500–2
Palmo-plantar nodular lesions in lepromatous leprosy
To the Editor:
Mycobacterium leprae has the unique ability among all mycobacteria to utilize DOPA by virtue of the enzyme diphenol oxidase (10). Cells of neural crest origin, e.g., Schwann cells, cells of the adrenal medulla and perineurial cells, can convert tyrosine to DOPA, offering an explanation for unusual tissue specificity of M. leprae (10). Another special feature of this organism is that it possesses a distinct predilection for the cooler portions of the body (2,3,8). Brand (3) observed that tendons, cartilage and bones are subject to active infection by M. leprae only when they lie close to the skin where the temperature is low. Based on this concept, the axilla, groin, perineum and narrow transverse band of skin over the lumbosacral region are described as "immune zones" for M. leprae because of their relative warmth (4). Even though palmoplantar skin is not included in the "immune zones," its involvement in leprosy is comparatively rare (1,9,11). All earlier reports on palmo-plantar involvement are in paucibacillary cases (9,11) and in only a single case of histoid leprosy (1). However, the literature is silent on lepromatous leprosy in this regard. In this communication we are reporting on a lepromatous leprosy patient with nodular lesions over the palms and soles.
A 40-year-old male presented to us with "glove-and-stocking" anesthesia and nodular infiltration over the palms, soles and back of elbows (Figs. 1 and 2). The nodules were shiny, smooth, well-demarcated, and 1-2 cm in size, forming almost confluent plaques. The patient also had a few ill-defined partial anesthetic patches on his back and buttocks, infiltration of both earlobes (Fig. 3) and loss of the lateral third of his eyebrows. The ulnar, common peroneal and greater auricular nerves were enlarged bilaterally and nontender. A bacterial index by slit-skin smear was 5+ from the earlobe, eyebrows and one of the nodules. Histopathology from nodular lesions was consistent with lepromatous leprosy.
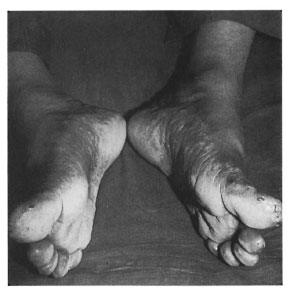
Fig. 1. Feet of patient showing plantar nodule lesions.
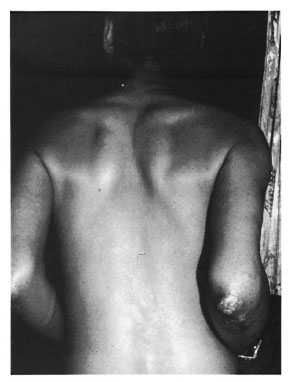
Fig. 2. Back view of patient showing nodules onhack of elbow.
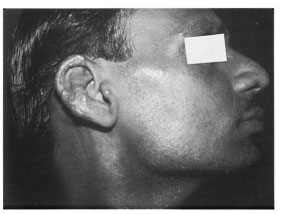
Fig. 3. Side view of patient showing nodular infiltration of earlobe.
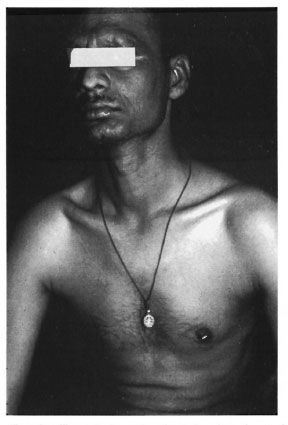
Fig. 4. Frontal view of patient showing chest rel-atively free from lesions.
Sabin, et al. (12) have shown that the temperature of the nerve bed is directly related to the depth of the tissues. There is a fairly good amount of nbro-fatty tissue on the palms and soles which ensures an insulating property and, hence, a high nerve bed temperature (7). The epidermis is also thicker on the palms and soles compared to other superficial skin areas and, hence, more warm (4). These are probably the reasons for infrequent involvement of the palms and soles in leprosy. However, in our patient the palms and soles were predominantly involved and the classical sites of lepromatous leprosy, including the back, chest, thighs, arms and abdomen, were relatively free. It is possible that the temperature dependency of M. leprae is not absolute, as evident by the fact that M. leprae survive in warm sites such as bone marrow (13), lymph nodes (6), and liver (5). This reasoning may partly explain the paradoxical distribution of lesions in our patient.
- Uma Shanker Agrawal
Puneet Bhargava
Ram Gulati
Narender Kumar Mathur
Department of Dermatology
SMS Medical College
Jaipur, India
REFERENCES
1. BASLAS, R. G., GUPTA, M., ARORA, S. K., MUKHIJA, R. D. and MISRA, R. K. Palmar involvement in histoid leprosy. Indian J. Lepr. 64 (1992) 193-195.
2. BINFORD, C. H. Comprehensive program for inoculation of human leprosy into laboratory animals. Public Health Rep. 71 (1956) 995-996.
3. BRAND, P. W. Temperature variation and leprosy deformity. In: VI! Transactions of the International Congress of Leprosy, Tokyo, 1958. Tokyo: Japanese Leprosy Foundation, 1959, pp. 125-129.
4. COCHRANE, R. G. Signs and symptoms. In: Leprosy in Theory and Practice. 2nd edn. Cochrane, R. G. and Davey, T. F., eds. Bristol: John Wright &Sons, 1964, p. 266.
5. CONTRERAS, J., JR., TERENCIO DE I.AS AGUAS, J. and CONTRI-IRAS, F. Hepatic lesions in lepromatous patients. Int. J. Lepr. 37 (1969) 270-279.
6. DESIKAN, K. V. and Jon, C. K. A review of postmortem findings in 37 cases of leprosy. Int. J. Lepr. 36 (1968)32-44.
7. ENNA, C. D., BERGHTHOLDT, H. T. and STOCK-WELL, F. A study of surface and deep temperatures along the source of the ulnar nerve in the pisohamate tunnel. Int. J. Lepr. 42 (1974) 43-47.
8. FELDMAN, W. H. Inducing pathogenesis of M. leprae in animals. Public Health Rep. 71 (1956) 995.
9. MUKHERJEE, N., GHOSH, S. and KUNDU, S. Palmar lesion in a case of leprosy of the tuberculoid type. (Abstract) Int. J. Lepr. 29 (1958) 245.
10. PRAHHAKARAN, K. Unique metabolic properties of Mycobacterium leprae. Indian J. Lepr. 63 (1991)410-417.
11. RAJKNDRA, N. Palmo-plantar lesions in paucibacillary leprosy-unusual clinical expressions. IndianJ. Lepr. 59(1987) 188-190.
12. SABIN, T. D., HACKIÍTT, E. R. and BRAND, P. W. Temperatures along the course of certain nerves often affected in lepromatous leprosy. Int. J. Lepr. 42(1974) 38-42.
13. SUSTLR, S., CABELLO-INCHAUSTI, B. and ROBINSON, M. J. Nongranulomatous involvement of bone marrow in lepromatous leprosy. Am. J. Clin. Pathol. 92(1990) 797-801.
Reprint requests to Dr. N. K. Mathur, C-24 Peeyush Path, Bapunagar, Jaipur 302 105, India.