- Volume 65 , Number 4
- Page: 497–500
Immunological reactions to mycobacterial proteins in the spectrum of leprosy
To the Editor:
Leprosy, still a public health probelm in many parts of the world, is a chronic mycobacterial disease produced by an intracellular parasite, Mycobacterium leprae, which multiplies mainly within tissue macrophages and Schwann cells in peripheral nerves. A good immunological response toward M. leprae depends on the development of a T-cell-mediated immune response.
It has not been established whether individual proteins are sufficient for the development of protective immunity in mycobacterial infections. Also, the availability of purified and well-charaterized antigens is a prerequisite for studying the role of individual molecules in the pathogenesis of the disease.
We have previously studied the effect of soluble M. leprae extract on the proliferative response of peripheral blood lymphocytes from leprosy patients, their family members and other contacts. In tuberculoid patients and in contacts we detected differential reactivity to the various proteins isolated (9).
Using molecular biology techniques and monoclonal antibodies, it has been possible to obtain individual proteins from mycobacterial antigens (1,2,6,8,15) wich has been very helpful for understanding the biology of this microorganism and its relationship with the host.
In this study, we have compared cellular and humoral reactivity toward various whole, fractionated and recombinant mycobacterial antigens from M. bovis and M. leprae in patients from the outpatient clinic of the Instituto de Biomedicina, Caracas, Venezuela. These patients were clinically and histologically diagnosed according to Ridley and Jopling's classification (11). The group included 20 lepromatous (LL) and borderline lepromatous (BL) patients and 20 tuberculoid (TT) patients, as well as a group of 10 family and nonfamily Mitsudapositive contacts. All LL and BL (85% males, 15% females) and TT (40% males, 60% females) patients were adults.
The antigens used for this study were: bacilli from experimentally infected armadillo lesions purified using Draper's method, BP (4); soluble M. leprae extrac from the same type of bacilli, ruptured in a French pressure cell (3); M. bovis BCG (Connaught Laboratories, Willowdale, Ontario, Canada); M. tuberculosis purified protein derivative (PPD) (Statens Seruminstitutet, Copenhagen, Denmark); and recombinant antigens 70 kDa from M. tuberculosis, 65 kDa from M. bovis, and 36 kDa, 28 kDa, 18 kDa, and 10 kDa from M. leprae. These recombinant antigens were obtained from the Recombinant Protein Bank of the World Health Organization. All soluble antigens were used at a 20-µg/ml final concentration in proliferation assays. Phytohemagglutinin (PHA) (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) was used as the positive control at a 25-µg/ml final concentration.
Lymphocyte transformation assay. Mononuclear cells from 20 ml of peripheral blood were obtained through a density gradient, diluted, and placed in flat-bottom, 96well plates (Falcon 3072; Falcon Plastics, Oxnard, California, U.S.A.) at 2 x 105 lymphocytes per well. Cell proliferation was measured as previously reported (9). Results were reported as the percentage positivity, and an index higher than 2 was considered positive. The various percentage positivities in the spectrum, with both recombinant proteins and complex antigens are shown in Tables 1 and 2.
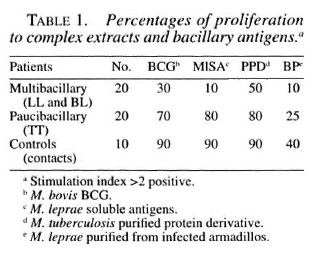
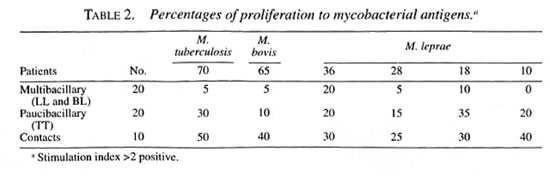
Multibacillary patients (18/20) did not show in vitro cellular reactivity to the recombinant antigens used, with a mean stimulation index of 0.98 ± 0.30, similar behavior to that shown with soluble extract (M1SA); only two showed cellular reactivity with M. tuberculosis 70 kDa and M. bovis 65 kDa, with a stimulation index of 2.26 ± 0.67 and 2.24 ± 0.85, respectively. These patients presented clinical spontaneous reversion. Paucibacillary patients showed reactivity with all of the recombinant proteins, more intense with M. leprae 18-kDa, M. tuberculosis 70-kDa, and M. leprae 10kDa proteins. Healthy family or nonfamily contacts reacted toward all of the antigens used. The highest values were obtained with M. tuberculosis 70-kDa, M. bovis 65kDa, and M. leprae 10-kDa proteins.
Studies done in Nepali leprosy patients and healthy controls showed strong correlation between BCG and M. leprae antigens. Patients and controls did not respond toward complex preparations nor toward recombinant antigens (12).
We had similar results in lepromatous patients, who lack T-cell responses, toward M1SA and recombinant antigens. T cells of tuberculoid patients and positive contacts recognized BCG/PPD in 70%/80% and 90%/90% of cases, respectively. Positive contacts recognized recombinant 70-kDa and 65-kDa proteins; in tuberculoid patients there were deficiencies in the cell-mediated response, it being much greater with the 70kDa protein (Table 2).
Work done in an endemic region of Ethiopia with 24 tuberculoid and 18 lepromatous patients and 21 healthy contacts using 11 antigenic molecules including M. leprae heat-shock protein (hsp) 10 and hspl8 and hsp65 did not show any association between the antigen-specific cellular response and the status of the various patients and controls studied (13).
Concerning cellular immunity, it is evident that the complete M. leprae protein extract is more immuogenic than individual proteins; if we study the percentage of positivity in the lymphocyte transformation tests of paucibacillary patients and family or nonfamily contacts, it is clear that several proteins are undoubtedly involved in the immune response. The stimulation index of T lymphocytes from lepromatous patients toward M1SA varied between 0.55, and 1.52; in tuberculoid patients the index varied between 1.72 and 10.02; in contacts, between 2.21 and 39.82.
ELISA study. IgG antibodies directed toward recombinant proteins were determined in an enzymatic assay (10). The values of circulating IgG antibodies directed toward complete extracts (cytosol and cell wall) and recombinants from M. leprae and M. tuberculosis were expressed in optical density (OD). Microtiter plates were sensitized with 5 µg/ml and 20 µg/ml recombinant antigens and 2 µg/ml and 10 µg/ml soluble M. leprae extract, 50 µl per well; one well was left with no antigen and two wells with antigen for each serum pool (LL and BL, T, and contacts) (14). Preliminary results using serum pools in the leprosy spectrum are shown in Table 3.
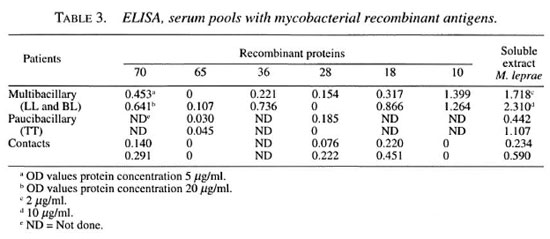
IgG antibodies responded in greater proportion to complex MISA and M. leprae 10-kDa protein, and in decreasing order to M. tuberculosis 70, M. leprae 18, and M. leprae 36 kDa. With M. bovis 65 kDa it was not possible to obtain good detection in the groups studied. Other workers have found that in the majority of leprosy patients and their contacts, IgG reacted to both of the 70kDa proteins from M. tuberculosis and M. bovis (BCG) (7). Quantitative differences found in this study using a pool of sera from patients and contacts are not clear. At present, we are developing sequential studies before, during and after multidrug therapy in individual patient's sera.
- Elsa Maria Rada, M.Sc.
Nacarid Aranzazu, M.D.
Jacinto Convit, M.D.
Instituto de Biomedicina
Laboratorio de Leprologia
Apartado 4043
Caracas 1010A, Venezuela
Acknowledgment. The authors acknowledge the collaboration granted by the WH O Bank of Recombinant Proteins in providing us with mycobacterial proteins. They also appreciate the collaboration given by Dr. Marian Ulrich for her helpful suggestions regarding the contents of the study.
REFERENCES
1. BOOM, R. J., HARRIS, D. P., LOVE, J. M. andWATSON, J. D. Antigenic properties of Mycobacterium leprae, complete sequence of the gene forthe 18 kDa protein. J. Immunol. 140 (1988)597-601.
2. BUCHANAN, T. M., NOGAGUCHI, H., ANDERSON, D. C., YOUNG, R. A., GILLIS, T. P., BRITTON, W. J.,IVANJI, J., Kali:, A. H., Cinss, 0., Bwom, B. R.and MEHRA, V. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of My-cobacterium leprae. Infect. Immun. 55 (1987)1000-1003.
3. CONVIT, J., ARANZALU, N., ULRICH, M., PINARDI, M. E., REYES, 0. and AINARADO, J. Iminunotherapy with a mixture of Mycobacterium leprae andBCG in different forms of leprosy and Mitsuda-negative contacts. Int. J. Lepr. 50 (1982)415-424.
4. DRAPER, P. Protocol 1/79: Purification of M. leprae . Report of the enlarged Steering Committee for Research on the Immunology of Leprosy(IMMLEP) Meeting of 7-8 February 1979.Geneva: World Health Organization, 1979, Annex 1, p. 4.
5. KAPLAN, G. and COHN, Z. A. The immunobiologyof leprosy. Int. Rev. Exp. Pathol. 28 (1986) 45-78.
6. KLATSER, P. R., Di: WITT, M. Y. and KOLK, A. H. J.An ELISA-inhibition test using monoclonal anti-body for the serology of leprosy. Clin. Exp. Immunol. 62 (1985) 468-473.
7. LAUNOIS, P., NIANG, M. N., DROWART, A., VANVOOREN, J. P., SARTHou, J. L., LALU, T., MII.I.AN,J. and HUYGEN, K. IgG response to purified 65-and 70-kDa mycobacterial heat shock proteins andto antigen 85 in leprosy. Int. J. Lepr. 62 (1994)48-54.
8. MEHRA, V., BLOOM, B. R., BAJARDI, A. C., GRISSO, C. L., SILTING, P. A., ALLAND, D., CONVIT, J., FAN,X.-D., HUNTER, S. W., BRENNAN, P. J., REA, T. H.and MooriN, R. L. A major T cell antigen of Mycobacterium leprae is a 10 kD heat-shock cognateprotein. J. Exp. Med. 175 (1992) 275-284.
9. RADA, E., SANTAELLA, C., ARANZAZU, N. and CONVIR, J. Preliminary study of cellular immunity to Mycobacterium leprae protein in contacts and leprosy patients. Int. J. Lepr. 60 (1992) 189-194.
10. RADA, E., ULRIC'', M., ARANZAZU, N., SANTAELLA,C., GALLINWO, M. E., CENTENO, M., RODRIGUEZ, V. and CONVIT, J. A longitudinal study of immunologic reactivity in leprosy patients treatedwith immunotherapy. Int. J. Lepr. 62 (1994)552-558.
11. RIDLEY, D. S. and JOLTING, W. H. Classification ofleprosy according to immunity; a live-group system. Int. J. Lepr. 34 (1966) 255-273.
12. ROCHE P. W., THEUVENET, W. J. and BRITON, W. Cellular immune responses to mycobacterial heatshock proteins in Nepali leprosy patients and con-trols. Int. J. Lepr. 60 (1991) 36-43.
13. THOLE, J. E. R., JANSON, A. A. M., KIFLE, A.,HOWE, R. C., MCLEAN, K., NURILYGN, A., ELLEN,E., SHANNON, E. J., BULL.A, G. J., HERMANS, J., DEVRIES, R. R. P., F ROMMEI., D. and RINKE DE WIT,T. Analysis of T-cell and B-cell responses to recombinant M. leprae antigens in leprosy patientsand healthy contacts, significant T-cell responsesto antigens in M. leprae nonresponders. Int. J.Lepr. 63 (1995) 369-380.
14. ULRICH, M., SMITH, P., SAMPSON, C., ZUNIGA, M.,CENTENO, M., GARCIA, V., MANRIQUE, X., SALGADO, A. and CONVIT, J. IgM antibodies to nativephenolic glycolipid-1 in contacts of leprosy pa-tients in Venezuela; epidemiological observationsand a prospective study of the risk of leprosy. Int.J. Lepr. 59 (1991) 405-415.
15. YOUNG, D. B., KAUFMANN, S. H. E., HERMANS, P. W. M. and THOLE:, J. E. R. Mycobacterial proteinantigens. Mol. Microbiol. 6 (1992) 133-145.