- Volume 64 , Number 1
- Page: 1–5
Detection of Mycobacterium leprae in nerve lesions by the polymerase chain reaction
ABSTRACT
A simple procedure is described for the detection of Mycobacterium leprae by the polymerase chain reaction in nerve biopsies sectioned with a cryostat and then treated with proteinase K. All samples f rom lepromatous leprosy patients and the majority of samples f rom paucibacillary cases yielded positive results. This approach may be useful for differentiating between leprosy and other inflammatory neuropathies.RÉSUMÉ
On décrit un procédé simple pour la détection de Mycobacterium leprae par la réaction de polymerase en chaîne dans un nerf biopsie sectionné par un cryostat et traité ensuite par proteinase K. Tous les échantillons provenant de patients lépromateux et la majorité des échantillons provenant de cas paucibacillaires ont donné des résultats positifs. Cette approche peut être utile pour différencier la lèpre d'autres neuropathies inflammatoires.RESUMEN
Se describe un procedimiento para la detección de Mycobacterium leprae por la reacción en cadena de la polimerasa en secciones de biopsias de nervios pretratadas con proteinasa K. Todas las muestras de los pacientes lepromatosos y la mayoría de los pacientes paucibacilares dieron resultados positivos. Este enfoque puede ser útil para diferenciar entre la lepra y otras neuropatías inflamatorias.Leprosy, an important public health problem in many countries, is generally diagnosed by clinical examination or inspection of slit-skin smears by microscopy (2,5). Bacilli are easily detected morphologically in nerve and skin biopsies of lepromatous patients. By contrast, detection of bacilli is extremely difficult in tuberculoid leprosy and in some patients under treatment or with reversal reactions. Since the causative organism Mycobacterium leprae has proved refractory to all attempts at in vitro culture, the mouse foot pad model was developed (7). However, mouse inoculation is a timeconsuming and relatively insensitive method (6). Nerve lesions in the tuberculoid form of leprosy lack specificity since similar inflammatory lesions are observed in other inflammatory neuropathies, such as sarcoidosis, and chronic inflammatory and demyelinating polyneuropathy of unknown origin. For such lesions it would be extremely useful to have a sensitive method of detecting the M. leprae genome. In recent years, a number of laboratories have described procedures for the detection and identification of M. leprae that involve the polymerase chain reaction (PCR) (3,8,10). These have been applied to purified organisms or M. leprae in skin specimens (1,10), but have not yet been tested on nerve biopsies. We describe here a simple procedure that allows the reproducible detection of M. leprae DNA in proteinase K-treated nerve biopsies.
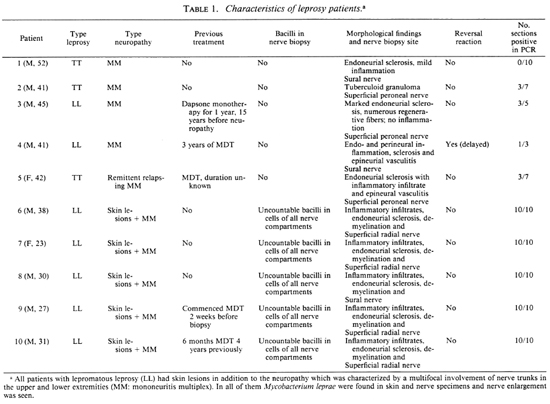
MATERIALS AND METHODS
Subjects. Nerve biopsy specimens from seven lepromatous patients and three paucibacillary patients were obtained from the Institut de Léprologie Appliquée of Dakar (Senegal) and the Pavillon de Malte, Hôpital Saint-Louis, Paris. Biopsies were taken from areas of sensory loss and, in the case of paucibacillary leprosy, no skin lesions were apparent. Two of the patients had received multidrug therapy previously (further details may be found in The Table). After Ziehl-Neelsen staining, morphological studies of paraffin-embedded and semithin sections enabled us to classify sections according to the presence of bacilli. Two nerve specimens from patients with inflammatory lesions due to cither necrotizing arteritis of the polyarteritis nodosa type or to sarcoidosis were used as negative controls.
Nerve samples. After the patient had given informed consent, nerve samples were taken by biopsy under local anesthesia and cut into three fragments: one was frozen (for PCR), one fixed in glutaraldehyde (for microscopy) and the third fixed in paraformaldehyde (PFA) (for immunology). The best PCR results were obtained when nerves were cut into ~10 -µ m sections using a cryostat (Frigocut, Reichert-Jung). Five to 10 sections were used for each patient. When possible, frozen and not fixed sections were used for reactions. To reduce the risk of contamination, the cryostat blades were replaced after each sample.
Preparation of samples for PCR. Sections from frozen or PFA-fixed specimens were resuspended in 100 µ d of buffer (60 raM Tris HC1, pH 8.8). In the optimized protocol, sections were treated with proteinase K at 50ºC for 30 min then heated for 5 min at 95ºC before use for PCR. Ten-Mi aliquots were used directly for PCR reactions. Proteinase K and collagenase/dispase were from IBoehringer Mannheim.
PCR reaction. Reactions were performed with primers R5 (TCACGCTTC-CTGTGCTTTGC) and R6 (TGCGCTA-GAAGCTTGCCGTA) directed against the M. leprae-speeihc repetitive sequence RLEP (9) which yielded a 447 base-pair (bp) fragment using the conditions described previously (10). Taq polymerase was purchased from Pcrkin Elmer Cctus. Amplification products were visualized by ethidium-bromidc staining after electrophoresis of 10- µ 1 samples on 1% agarose gels. The positive control was 0.1-10 ng of a plasmid pSWl 1 carrying a deleted-RLEP derivative that yields a product of 250 bp. Negative controls were reaction mixes containing all reagents but no DNA or biopsy samples from patients with necrotizing arteritis or sarcoidosis prepared by the same technique.
RESULTS
Optimization of DNA extraction from nerves. A major problem in applying PCR to the detection of M. leprae in nerve biopsies is the initial lysis step since the frequently extensive fibrosis of nerves, in both paucibacillary or multibacillary leprosy, makes DNA extraction very difficult. Consequently, several different lysis procedures, both mechanical and enzymatic, were attempted. In the initial experiments, PCR reactions were performed on freeze-boiled (10) samples, from entire nerve preparations (~3-5 mm in length), from a lepromatous leprosy patient, after mechanical teasing, with or without collagenase or collagenase/ dispase treatment. The results (not shown) were poorly reproducible and collagenase/ dispase was shown to contain inhibitors of Taq polymerase.
It was subsequently found that satisfactory results were obtained when sections of nerves were cut to a ~10- µm thickness with a cryostat and then processed for PCR. Furthermore, treatment of the nerve sections with proteinase K for 30 min prior to freezeboiling (10) increased the reproducibility and sensitivity considerably. The Figure shows that when PCR was performed on serial dilutions of sections from the same nerve, with or without proteinase K treatment, the sensitivity was increased by 50- to 100-fold after proteinase K treatment.
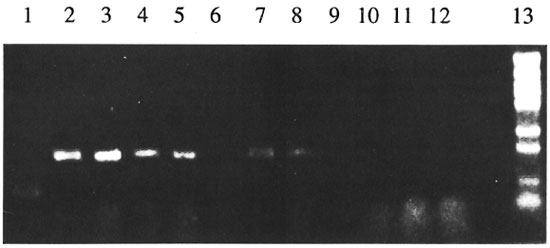
The Figure. Comparison of sensitivity of detection of M. leprae DNA in nerve specimens with or withoutproteinase K treatment. Frozen sural nerve sections from a lepromatous patient (#8, The Table) were suspendedin Tris buffer with or without proteinase K and diluted 10- to 104-fold. Aliquots (10 Ad) from the dilutedsuspension were used for PCR then analyzed by agarose gel electrophoresis. Treated and untreated samples werein lanes 2-6, and 7-11, respectively. Lane 1 = positive control (pSW11); lane 2 = undiluted; lane 3 = 10-folddilution; lane 4 = 100-fold dilution; lane 5 = 1000-fold dilution; lane 6 = 10,000-fold dilution; lane 7 =undiluted; lane 8 = 10-fold dilution; lane 9 = 100-fold dilution; lane 10 = 1000-fold dilution; lane 11 = 10,000-fold dilution; lane 12 = negative control; lane 13 = molecular weight markers.
Detection of M. leprae by PCR in various nerve biopsies and correlation with morphological findings. To evaluate the usefulness of this approach, the optimized extraction procedure was applied to nerve biopsies from 10 well-characterized leprosy patients with mononeuritis multiplex (The Table) who were classed in two groups. The first group (patients 6-10) represented five cases of lepromatous leprosy, and large numbers of acid-fast bacilli were visible in cells of all nerve compartments. PCR signals were generally obtained after 30 cycles of amplification with all samples tested. In the second group, which contained three tuberculoid patients and two previously treated lepromatous patients (patients 1-5), no bacilli were seen by either light or electron microscopy and 35 to 40 PCR cycles were required to generate products. However, positive results were only obtained from 4 of the 5 patients, and even then only ~35 % of the sections assayed generated a PCR product. In one tuberculoid patient, all samples were negative although there was no indication of the presence of inhibitors.
DISCUSSION
The type of neuropathy observed in leprosy depends upon the host's cellular immune response to M. leprae. Reversal reactions (type 1) may lead to further damage to the nerve without morphologically detectable bacilli. Here, a sensitive PCR-procedure for detecting M. leprae in nerve biopsies was developed, optimized and applied to nerve biopsies from patients with different forms of leprosy. As expected, all samples examined from lepromatous leprosy patients tested positive; whereas only 50% of the sections assayed from nerves, in which no bacilli were visible by light or electron microscopy, yielded positive results. This group included a patient presenting with reversal reactions. In the case of one tuberculoid patient, none of the samples was positive in the PCR assay.
Detection of M. leprae in nerves by PCR is more sensitive than by microscopy, although there is a certain variability in paucibacillary patients. This may be due to the small number of bacilli present in lesions and to the focal nature of the lesions in these patients. Our findings are consistent with the results of de Wit, et at. (1) in skin biopsies in which 56% of tuberculoid samples were PCR positive. In reversal reactions, the detection of bacterial DNA in the absence of morphologically detectable bacilli does not necessarily mean the presence of viable bacilli, since in recently treated patients, M. leprae DNA was found to persist long after the death of the organisms (1,4). PCR may be of help to differentiate leprosy from other inflammatory neuropathies, and further studies are in progress.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Additional financial assistance was generously provided by the Association Française Raoul Follereau, the Institut National de la Santé et de la Recherche Médicale (CRE 910604 and 920813), Contrat de Recherche Clinique (910610), and the Institut Pasteur.
REFERENCES
1. DE WIT, M. Y. L., FABER, W. R., KRIEG, S. R., DOUGLAS, J. T., LUCAS, S. B., MONTREEWASUWAT, N., PATTYN, S. R., HUSSAIN, R., PONNINGHAUS, J. M., HARTSKEERRL, R. A. and KLATSER, P. R. Application of a polymerase chain reaction for the detection of Mycobacterium leprae in skin tissues. J. Clin. Microbiol. 29 (1991) 906-910.
2. DHARMENDRA. Classifications of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 88-99.
3. HARTSKEERL, R. A., DE WIT, M. Y. L . and KLATSER, I. R. olymerase chain reaction for the detection of Mycobacterium leprae. J. Gen. Microbiol. 135 (1989) 2357-2364.
4. JAMIL, S., KEER, J., LUCAS, S. B., DOCKRELL, H. M., CHIANG, T. J., HUSSAIN. R. and STOKER, N. G. Use of polymerase chain reaction to assess ellicacy of leprosy chemotherapy. Lancet 342 (1993) 264-267.
5. RIDLEY, D. S. and JOB, C. K. The pathology of leprosy. In: Leprosy. R. C. Hastings, ed. Edinburgh: Churchill Livingstone, 1985. pp. 100-133.
6. SHEPHARD, C. C. Experimental leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 269-286.
7. SHEPHARD, C. C. and CHANG, Y. T. Effect of several anti-leprosy drugs on multiplication of human leprosy bacilli in footpads of mice. Proc. Soc. Exp. Biol. Med. 109 (1962) 636-638.
8. WILLIAMS, D. L., GILLIS. T. P., BOOTH, R. J., LOOKER, D. and WATSON, J. D. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J. Infect. Dis. 162 (1990) 193-200.
9. WOODS, S. A. and COLE. S. T. A family of dispersed repeats in Mycobacterium leprae. Mol. Microbiol. 4 (1990) 1745-1751.
10. WOODS, S. A. and COLE, S. T. A rapid method for the detection of potentially viable Mycobacterium leprae in human biopsies: a novel application of PCR. FEMS Microbiol. Lett. 65 (1989) 305-310.
1. M.D., Service de Neurologie-Adulte, Centre Hospitalier Universitaire de Bicetre, 78 Rue du General Leclerc, 94275 Le Kremlin Bicetre, France.
2. Ph.D., Unite de Génétique Moléculaire Bactérienne, Institut Pasteur, 28 Rue du Docteur Roux, 75724 Paris 15, France.
Reprint requests to Dr. Cole at address above or Tel. 33-1-45688446; Fax. 33-1-45698593.
Present address for Dr. Woods: Pharmacia Biotech, Parc Technologique, 91898 Orsay, France.
Received for publication on 17 July 1995.
Accepted for publication in revised form on 31 October 1995.