- Volume 64 , Number 1
- Page: 6–14
Immunological profiles of leprosy patients and healthy family contacts toward M. leprae antigens
ABSTRACT
In this study, we measured simultaneously the in vitro and in vivo T lymphocyte reactivities and the antibody responses of leprosy patients and healthy family contacts (HFC) toward Mycobacterium leprae antigens. The in vitro lymphoproliferativc response of the HFC to leprosin A was comparable to that of tuberculoid leprosy patients. However, their skin-test reactivity to Dharmcndra lepromin was considerably higher compared to the in vitro response to leprosin A. A significant number of HFC failed to respond to M. leprae antigens, both in vitro and in vivo, and the unresponsiveness to either test was not related to the type of leprosy patients in the household. A marginal correlation was observed between the skin-test reactivity of HFC and the age of the individuals. Even though a significant proportion of HFC showed positive anti-PGL-I IgM levels, none showed a positive titer in the serum antibody competition test toward the M. leprae-specihe epitope My2. A positive anti-PGL-I IgM response together with a negative lepromin skin-test reactivity showed a clear downward trend f rom the lepromatous pole toward the tuberculoid pole. A small number of HFC, all contacts of lepromatous patients, were lepromin skin-test negative with positive anti-PGL-I IgM levels, but the majority among them showed T-cell reactivity to mycobacterial antigens in vitro. These results are discussed in relation to immunological correlates of the susceptibility to M. leprae infection.RÉSUMÉ
Dans cette étude, nous avons mesuré simultanément la réactivité in vitro et in vivo des lymphocytes T et les réponses en anticorps de malades de la lèpre et de contacts familiers en bonne santé vis-à-vis des antigènes de Mycobacterium leprac. La réponse de prolifération lymphocytaire in vitro des contacts vis-à-vis de la léprosinc A était comparable à celle des patients présentant une lèpre tuberculoide. Cependant, leur réactivité au test cutané avec la lépromine de Dharmendra était considérablement plus élevée que la réponse in vitro vis-à-vis de la léprosine A. Un nombre significatif de contacts ne répondait pas aux antigènes de M. leprae. aussi bien in vitro qu'in vivo, et il n'y avait pas d'association entre leur non-réponse aux tests et le type de lèpre du malade dont ils étaient les contacts. Une correlation faible a été observée entre la réactivité des contacts au test cutané et l'âge des individus. Même si une proportion significative des contacts a montré des taux positifs d'IgM anti-PGL-I, aucun n'a montré un titre positif dans le test de compétition d'anticorps sériques vis-à-vis de l'épitope My2 spécifique de M. leprae. Une réponse positive d'IgM anti-PGL-I associée à un test cutané à la lépromine négatif, montrait une nette tendance à la diminution depuis le pole lépromateux jusqu'au pole tuberculoide. Un petit nombrae de contacts, tous contacts de malades lépromateux, présentait un test cutané à la lépromine négatif ainsi que des taux positifs d'IgM anti-PGL-I, mais la majorité d'entre eux montrait une réactivité des cellules T vis-à-vis des antigènes mycobactériens. Ces résultats sont discutés en rapport avec les correlations immunologiques de la susceptibilité visà-vis de l'infection à M. leprae.RESUMEN
En este estudio se midieron simultáneamente las reactividades in vitro e in vivo de los línfocitos T, y las respuestas en anticuerpos de pacientes con lepra y sus convivientes sanos (CS) contra antígenos de Mycobacterium leprac. La respuesta Iinfoproliferativa in vitro de los CS a la leprosina A fue comparable a la de los pacientes con lepra tuberculoide. Sin embargo, su reactividad en piel a la lepromina de Dharmendra fue considerablemente mayor que la respuesta in vitro a la leprosina A. Un número considerable de CS no respondieron a los antígenos de M. leprac, ni in vitro ni in vivo, pero esto no estuvo relacionado con el tipo de lepra del paciente conviviente. Se observó una correlación marginal entre la reactividad en piel de los CS y la edad de los individuos. Aún cuando una importante proporción de CS tuvieron niveles mcdibles de anticuerpos IgM anti-PGL-I, ninguno mostró anticuerpos contra el epitopo My2 específico de M. leprae. La presencia de anticuerpos IgM anti-PGL-I y la reactividad en piel negativa a la lepromina, mostraron una clara tendencia a revertir del extremo lepromatoso al extremo tuberculoide de la enfermedad. Un pequeño número de CS, todos contactos de pacientes LL, fueron lepromino negativos y tuvieron anticuerpos IgM anti-PGL-I pero la mayoría de ellos mostraron reactividad celular T (in vitro) hacia antígenos micobacterianos. Estos resultados se discuten en relación a los factores inmunológicos de susceptibilidad a la infección por M. leprae.Leprosy continues to be a major public health problem in developing countries (20). The causative organism, Mycobacterium leprae, is almost avirulent (23) since more than 95% of the individuals living in endemic areas do not develop any clinical manifestation of the disease (6,33). In susceptible individuals who develop symptoms, the incubation period ranges from 3 months to 30 years, the average being 2-5 years (19). The clinical picture is varied from the tuberculoid to the lepromatous pole, which correlates well with the immunological status of the host toward M. leprae antigens (22). The presenting symptoms of leprosy are related to a) the multiplication and dissemination of M. leprae, b) the patients' immune response to M. leprae antigens, and c) the complications arising as consequences of these two processes. The host immune response toward M. leprae is complex (7), being both cellular and humoral in nature. Since M. leprae is an intracellular pathogen, it is the cell-mediated immunity (CM I) that confers protection (18). Under circumstances in which effective CMI fails to develop, M. leprae multiplies unchecked. Neither the host factors which determine the susceptibility of an individual to M. leprae nor the M. leprae factors that help in the successful establishment of the infection are completely understood (18).
The literature on the immune responses of leprosy patients and healthy contacts toward mycobacterial antigens is extensive (7). In addition to the efforts to achieve primary immunoprophylaxis against leprosy, the early diagnosis of established preclinical infection continues to be one of the main objectives in leprosy control programs (5). The M. leprae -specific serological assays, phenolic glycolipid-I (PGL-I) ELISA and scrum antibody competition test (SACT) had only limited success in achieving this goal (26,31). However, a few studies have evaluated more than one immunological parameter simultaneously, for example, the lepromin skin test with a serological assay (1,2,10). In view of the complexity of the immune response toward M. leprae antigens, it was felt essential to study the contact population for both cell-mediated and humoral immune responses simultaneously, toward a more precise definition of their immunological status against M. leprae antigens. In the present study, we have evaluated the in vivo and the in vitro T-cell responses of the healthy household contacts of leprosy patients together with two M. leprae-spcciiic serological assays, and compared them with those of the patients.
MATERIALS AND METHODS
Antigens. M. leprae sonicate antigen (leprosin A, batch CD91), natural disaccharide of PGL-I conjugated to BSA (ND-O-BSA), and unconjugated BSA of the same lot were kind gifts from Dr. R. J. W. Rees (WHO/IMMLEPAW. leprae bank). The Dharmendra preparation of M. leprae was obtained from Dr. U. Sengupta, Central JALMA Institute for Leprosy, Agra, India. The BCG (Danish strain 1331) was kindly provided by The Director, BCG Vaccine Laboratories, Madras, India. Purified protein derivative (PPD) was obtained from The Royal Veterinary Laboratories, Ridgeway, Mill Hill, London. Monoclonal antibody ML04 conjugated to horseradish peroxidase (ML04-HRP) was a gift from Dr. J. Ivanyi, Hammersmith Hospital, London.
Cell culture reagents. Hank's balanced salt solution (HBS), RPMI-1640, penicillinstreptomycin mixture and PH A-P were purchased from Sigma Chemical Co., St. Louis, Missouri, U.S.A. Ficoll-paquc was obtained from Pharmacia, Uppsala, Sweden.
Blood and serum samples. Blood samples from leprosy patients and healthy family contacts (HFC) were collected from a leprosy hospital (Voluntary Health Services, Leprosy project) located at Sakthinagar (Periyar District, Tamil Nadu, India). Leprosy patients were classified clinically and bacteriologically into polar lepromatous (LL), borderline lepromatous (BL), midborderline (BB), borderline tuberculoid (BT) and polar tuberculoid (TT) patients (22). The patients included cases under multidrug chemotherapy for periods varying from 2 weeks to 228 weeks and a few untreated subjects. LL and BL patients were grouped together as lepromatous patients. Those lepromatous patients who had become bacterial index (Bl)-negative (LBI-) following chemotherapy were distinguished from BI- positive lepromatous patients (LBI + ). HFC were healthy individuals living in the same household with the leprosy patients, and were thoroughly examined for any leprosy lesion. The study subjects were randomly selected without any bias toward age or sex. However, individuals younger than 12 years and older than 70 years of age were not included. Healthy noncontact (HNC) samples derived from the student community of the Madurai Kamaraj University, who had not had any habitual contact with leprosy patients (even though they lived in an endemic area), served as controls. A total of 260 samples which included 146 leprosy patients (56 LBI +, 18 LBI -, 18 BB, 32 BT, 22 TT), 67 HFC and 47 HNC were studied. Each subject donated about 20 ml of venous blood into heparinized vacutainers (Vacuette, Griener, Germany) and/or 4 ml into siliconized vacutainers for serum samples.
Lymphoproliferation assays. Peripheral blood mononuclear cells (PBMC) were separated over Ficoll-paque, washed with H BS, and suspended in RPMI 1640 containing 100 U/ml of penicillin, 100 µ g/ml of streptomycin and 10% human AB serum at a concentration of 1 x 106 cells per ml. Cultures with 105 cells were stimulated with leprosin A (10 µ g/ml), BCG (5 x 105 bacilli/ml) or PPD (20 µ g/ml). Triplicate cultures in 96-well, flat-bottom microtiter plates (Nunc, Roskilde, Denmark) were incubated for 6 days at 37ºC in a humidified atmosphere of 5% CO2 and 95% air. As a control, one set of the culture was stimulated with 10 µ g/ml PHA-P for 3 days in medium containing 10% FCS. During the last 16 hr of culture, 0.5 µ Ci of 3H-thymidine (Bhabha Atomic Research Centre, Bombay, India; specific activity 6.7 Ci/mmol) was added to each well. Cultures were harvested onto glass-fiber filters and the radioactivity incorporated was measured by a liquid scintillation counter (LKB Wallac, Turku, Finland).
Lepromin skin test. A lepromin skin test was performed on the same day blood samples were collected for lymphoproliferation assays. Indurations developed in response to intradermally inoculated Dharmendra lepromin (0.1 ml) were recorded 21 days post-inoculation (late lepromin reaction).
Serological assays. Serological tests were performed as detailed elsewhere (15). For anti-PGL-I IgM ELISA, microtiter plates (Corning Glass Works, Corning, New York, U.S.A.) were coated with 0.1 µ g/ml of ND-O-BSA of control BSA in carbonate buffer (pH 9.6) for 1 hr at 37ºC and then overnight at 4ºC. After blocking with 1% BSA in PBS containing 0.05% Tween 20 (BSA-PBST), the wells were incubated with 1:300 dilution of serum samples followed by horseradish peroxidase (HRP) conjugated anti-human IgM (Cappel, Belgium). After a thorough wash, the reaction was developed with a substrate solution containing 0.05% o-phenylenediamine (Sigma) and 0.012% hydrogen peroxide in citrate buffer pH 5.0, and the optical density (OD) was measured at 490 nm using a Dynatech Minireader II. For each sample, the mean OD of the BSA-coated wells was subtracted from that of the ND-O-BSA-coated wells. Antigen-coated wells without test serum served as blanks. Samples with OD values above the mean + 2 S.D. value of HNC (> 0.20) were considered positive.
The antibody response to a species specific epitope (My2) on the 35-kDa M. leprae protein was measured by inhibition of the specific monoclonal antibody (ML04-HRP) binding by serum antibodies (SACT). Each serum sample was tested over serial fivefold dilutions from 1:5 to 1:625. The binding of ML04-HRP to leprosin A-coated wells in the absence of serum gave the 100% binding value. The relative binding values of ML04-HRP in wells incubated with different serum dilutions were calculated using 100% binding value for each plate. The percent inhibition values were derived from these values, and the highest dilution of the test serum which showed more than 50% inhibition of the 100% binding value (ID5 0 titer) was determined. A serum sample was considered SACT positive when the ID50 titer was more than 1:5.
Statistical analysis. Student's / test and regression analysis were performed using the EPISTAT statistical package.
RESULTS
Healthy family contacts of leprosy patients, noncontacts, and tuberculoid leprosy patients showed significantly higher lymphoproliferative responses to leprosin A compared to lepromatous and midborderline patients (p < 0.05 to 104, Fig. 1). The responses of the HFC toward leprosin A were comparable to those of tuberculoid patients. Even though only 40% of the family contacts responded to leprosin A in vitro, a considerably higher proportion (60%) showed skin-test reactivity to Dharmendra lepromin (Fig. 1). A comparison between the lymphoproliferative response to leprosin A and the lepromin skin-test reaction did not show a significant correlation among the healthy contacts (Fig. 2). In fact, a large proportion of healthy contacts (40%) who failed to respond to leprosin A in vitro showed positive skin-test reactivity to Dharmendra lepromin. A similar trend was observed with tuberculoid patients as well (data not shown). About 23% of the contacts failed to respond to M. leprae antigens both in vitro and in vivo. Interestingly, a small proportion of the individuals (12%) who showed lymphoproliferative responses to leprosin A failed to develop lepromin reactions.
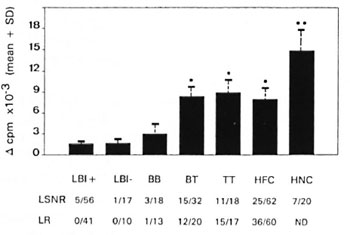
Fig . 1. In vitro and in vivo cellular immune responses of leprosy patients and healthy family contacts and controls toward M. leprae antigens: 1 x 105 PBMC were stimulated with 20 µ g/ml of leprosin A for 6 days. 3H-thymidine incorporated during the last 16 hr of culture is expressed as Δ cpm (mean cpm of stimulated culture - mean cpm of control culture with medium alone). LSNR = Proportion of rcspondcrs to leprosin A (LSN) in vitro with Δ cpm values >5000 and Stimulation Index (SI) (mean cpm of stimulated culture/ mean cpm of control culture with medium alone) >3.0. LR = Proportion of lepromin skin-test responders who developed > 3 mm of induration 21 days after the intradermal inoculation of Dharmendra lepromin (L). * = Mean value significantly high compared to LBI + , LBI- and BB patients. ** = Mean value significantly high compared to all the other groups. ND = Not done.
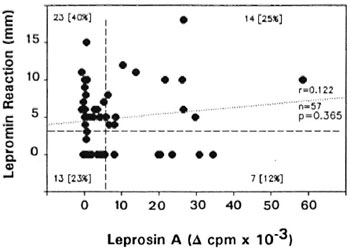
Fig. 2. Lack of correlation between lymphoproliferative response to leprosin A and skin-test reactivity among healthy contacts. Correlation between the Δ cpm values of in vitro response and the diameter of the lepromin reaction was evaluated by regression analysis. Vertical and horizontal dashed lines bisecting the x and y axes represent the respective positive cutoff values (for details see Fig. 1.). Number and percentage (in parentheses) of individuals within each quadrant are shown; r = correlation coefficient; n = total number contacts studied; p = significance level of correlation.
To investigate whether the T-cell reactivity of HFC toward M. leprae antigens is related to their relative exposure to M. leprae, they were segregated into two groups according to the source case, namely, contacts of lepromatous leprosy patients and contacts of tuberculoid leprosy patients. Comparison of in vivo or in vitro responses to M. leprae antigens did not show a significant difference between these two groups (data not shown). However, a marginal positive correlation was observed between the age of the individuals and the skin-test reactivity to Dharmendra lepromin (Fig. 3), but not the lymphoproliferative response to leprosin A (data not shown). Interestingly, the age of the lepromin nonresponders showed a very wide distribution (Fig. 3).
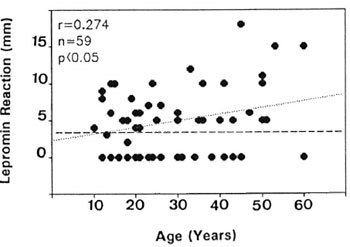
Fig . 3. Correlation between lepromin reactivity and age of individuals among healthy contacts. Horizontal dashed line bisecting the y axis represents the positive cutoff value for lepromin reactivity.
DISCUSSION
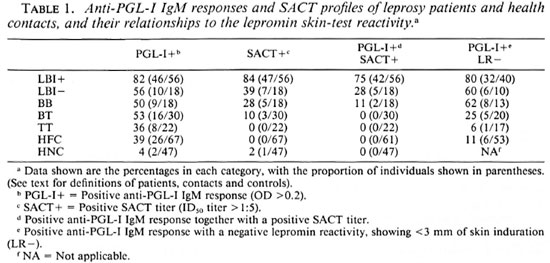
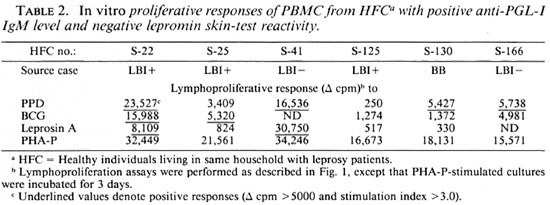
Studies on the antibody response toward M. leprae antigens revealed that a significant proportion of healthy contacts (39%) showed positive anti-PGL-I IgM levels (Table 1). However, none of these individuals showed seroreactivity toward the M. leprae specific epitope My2 in the serum antibody competition test, a trend also seen among tuberculoid patients. This is in contrast to the LBI+ group wherein 75% of the individuals were positive for both serological tests. Lepromatous patients who had become BI negative following chemotherapy and BB patients showed a considerably lower proportion of this dual seropositivity, reflecting a reduced bacillary load in their lesions. When the lepromin skin-test reactivity was considered together with the serological assays, a clear downward trend for a positive anti-PGL-I IgM response with a negative lepromin skin-test reactivity was evident from the lepromatous pole toward the tuberculoid pole (Table 1). Noticeably, about 11% (6 out of 53) of the contacts who had a positive anti-PGL-I IgM level also failed to develop lepromin skin-test reaction (Table 1). Evaluation of other data available on these six subjects revealed that most of them are contacts of lepromatous leprosy patients (Table 2). Interestingly, five of them showed discernible-to-moderate levels of T-cell reactivity to M. leprae and/ or BCG antigens in in vitro lymphoproliferation assays (Table 2).
The majority of individuals in leprosyendemic areas do not develop the disease. In most of the exposed individuals, the infection is largely subclinical presumably because of the development of an effective cellmediated immunity and self-healing. Only a few individuals actually develop clinical symptoms (approximately 2 per 1000), and the mechanisms underlying susceptibility or resistance to M. leprae infection remain poorly understood (18,33). Therefore, detailed investigations into the immunological correlates associated with the development of overt clinical symptoms among the healthy household contacts of leprosy patients would help to better understand the pathogenetic process leading to the establishment of the disease, and to provide a more precise definition of the high-risk individuals.
In the present work, 62 healthy family contacts of leprosy patients were studied for their immunological responsiveness toward mycobacterial antigens, both cellular and humoral. While about 60% of them were reactive to the lepromin skin test, only 40% showed in vitro lymphoproliferative responses to M. leprae antigens. A similar trend was observed in tuberculoid patients. This may probably be due to the fact that the antigen-specific T cells arc recruited to the skin-test site over a period of 3 weeks; whereas the lymphoproliferative response depends on the frequency of the reactive T cells in the peripheral circulation at the time of blood sampling, which may be influenced by factors other than the immune status of the host toward the antigen(s). Therefore, it is considered that the lepromin skin-test reaction, reflective of the individual's ability to mount a granulomatous reaction toward M. leprae, correlates well with the protective immunity (29). However, Sampaio, et al. ( 25) have shown that persistent in vitro unresponsiveness and poor gamma-interferon (IFN-γ) production toward M. leprae (leprosin A) are associated with the development of an active disease. Although the lepromin skin test is not diagnostic, it has been shown to have a prognostic value among contacts. In a large-scale study, evaluated after 15 years, Dharmendra and Chatterjee (11) observed that a significant proportion of the lepromin skin-tcst-negative contacts developed the lepromatous type of lesions. Similar observations were made by Ramu (21) among contacts including children. The proportion of lepromin skin-testpositive contacts in the present study could be an underestimation, because higher doses of antigen and repeated testing were shown to improve lepromin reactivity (11,12). A positive correlation between skin-test reactivity and the age of the contacts, but the lack of a significant difference between the contacts of lepromatous and tuberculoid patients in their lepromin reactivity, suggests that the cell-mediated immune response to M. leprae antigens might be influenced by factors other than the quantum exposure to M. leprae. In this context, it should be noted that exposure to environmental mycobacteria could significantly modulate the immune response to M. leprae (13).
In the present investigation, two M. leprae-specific serological assays were employed to study the humoral immune responses of healthy family contacts toward M. leprae. PGL-I ELISA (35) has been used widely to define high-risk individuals among contacts (31). In the present study, more than 35% of the healthy family contacts showed anti-PGL-I IgM antibody levels above the positive cutoff value. Several other studies on household contacts have shown varied results, with seropositivity ranging from 7% to 40% (31). Noncontact controls in endemic areas showed a much less (approximately 5%) seroprevalence rate. In the present work, noncontact controls from an area away from the study population showed only a 4.2% seropositivity. Repeated observations on healthy subjects in an endemic population have shown conversion from seropositivity to seronegativity and vice versa, while about 5% remained persistently seropositive over a period of 2 years (4). Several authors have observed that anti-PGL-I IgM levels in healthy individuals depend on the endemicity of the population (4,8) and varies with the prevalence rate of leprosy (9). The prevalence rate in our study population was 15.32 per 1000. A few prospective studies have demonstrated that an elevated anti-PGL-I IgM level per se is not diagnostic of individuals at risk (4-14-32).
The proportion of individuals with antibodies to the M. leprae-specific epitope of the 35-kDa protein showed a gradation across the leprosy spectrum, but none of the TT patients or the healthy contacts was positive, even though a considerable proportion among them had significant anti-PGL-I IgM levels. Variable proportions of SACT seropositivity have been reported among household contacts (3,17). It has been suggested that while anti-PGL-I IgM antibodies arise early during the course of infection SACT becomes positive well after the establishment of the disease (30). Therefore, it is likely that the anti-PGL-I IgM-positive contacts in this study might have had a subclinical infection which had either healed or had not yet become sufficiently established to stimulate antibody response against the 35-kDa protein antigen.
The majority among the contacts with a positive anti-PGL-I IgM response showed lepromin skin-test reactivity, suggesting that they have developed an effective CMI response to M. leprae. However, 6 out of 53 family contacts showed a positive anti-PGL-I IgM level as well and failed to develop lepromin reactions. Interestingly, two of them showed in vitro T-cell reactivity to M. leprae antigens and five to crossreactive mycobacterial antigens. Even though the observed lepromin nonreactivity could be an underestimation, other possibilities, such as M. leprae- specific suppressor T cells (24) or a differential regulation of the T-helper subsets (34), elicited by certain mycobacterial components in vivo (27) but too low in frequency to be effective in in vitro lymphoproliferative assays, may also be considered.
Earlier we had demonstrated that healthy family contacts, as a group, are distinct from noncontacts in terms of their immunological reactivity to M. leprae antigens (15,16,28). In this study, we have carried out a simultaneous evaluation of their cellular and humoral immune responses to M. leprae antigens. A limited number of studies have been carried out in populations at risk with simultaneous evaluation of the lepromin skin test, the FLA-ABS test, or anti-PGL-I ELISA (1,2,10). Even though there is some scepticism regarding the cost-effectiveness and the technical difficulties involved in the application of extensive immunological tests as diagnostic tools (26), their usefulness in understanding the early immunological events associated with the development of overt clinical symptoms of leprosy is indisputable. Therefore, it is worthwhile to undertake a similar, multiparameter, immunological evaluation of the healthy contacts in leprosy-endemic areas, which can be better achieved by integrating it into large-scale vaccine trials where the required machinery is already available.
Acknowledgment. This work was supported by grants from the Department of Biotechnology, the Government of India, and the Indo-Swiss collaboration in Biotechnology, Zurich. S.I. and S.R. thank the CSIR, the Government of India for senior research fellowships.
REFERENCES
1. ABE, M., O ZAWA, T., M INAGAWA, F. and Y OSHINO, Y. Immunoepidemiological studies on subclinical infection in leprosy. I. Clinical and immunological findings in school children and adults in Okinawa. Jpn. J. Lepr. 59(1990)130-144.
2. AGIS, F., S CHLICH, P., C ARTEL, J. L., GUIDI, C. and BACH, M.-A. Use of anti- M . leprae phenolic glycolipid-I antibody detection for early diagnosis and prognosis of leprosy. Int. J. Lepr. 56(1987)527-535.
3. ASHWORTH, M., SINHA, S., PATIL, S. A., RAMU, G. and SENGUPTA, U. The detection of subclinical leprosy using a monoclonal antibody based radioimmunoassay. Lepr. Rev. 57(1986)237-242.
4. BAGSHAWE, A. F., GARSIA, R. J., BAUMGART, K. and ASTBURY, L. IgM serum antibodies to phenolic glycolipid-I and clinical leprosy: two year's observation in a community with hyperendemic leprosy. Int. J. Lepr. 58(1990)25-30.
5. BAUMGART, K. W., BRITTON, W. J., MULLINS, R. J., BASTEN, A. and BARNETSON, R. ST.C. Subclinical infection with Mycobacterium leprae- a problem for leprosy control strategies. Trans. R. Soc. Trop. Med. Hyg. 87(1993)412-415.
6. BLOOM, B. R. Learning from leprosy: a perspective on immunology and the third world. J. Immunol. 137(1986)i-x.
7. BRITTON, W . J. Leprosy 1962-1992. 3. Immunology of leprosy. Trans. R. Soc. Trop. Med. Hyg. 87(1993)508-514.
8. CHANTEAU, S., CARTEL, J. L. GUIDI, C, PLICHART, R. and BACH, M.-A . Scroepidemiological study on 724 household contacts of leprosy patients in French Polynesia using disaccharidc-octyl-BSA as antigen. Int. J. Lepr. 55(1989)626-632.
9. CHO, S. N., KIM, S. H., CELLONA, R. V., CHAN, G. P., FAJARDO, T. T., WALSH, G. P., KIM, J. D. and BRENNEN, P. J. Prevalence of IgM antibodies to phenolic glycolipid I among household contacts and controls in Korea and The Philippines. Lepr. Rev. 63(1992)12-20.
10. DAVID, H. L., PAPA, F., CRUAUD, P., BERLIE, H. C, MAROJA, M. F., SALEM, J. I . and COSTA, M. F. Relationships between titers of antibodies immunoreacting against glycolipid antigens from Mycobacterium leprae and M. tuberculosis, the Mitsuda and Mantoux reactions, and bacteriological loads: implications in the pathogenesis, epidemiology and serodiagnosis of leprosy and tuberculosis. Int. J. Lepr. 60(1992)208-224.
11. DHARMENDRA and CHATTERJEE, K. R. Prognostic value of the lepromin test in contacts of leprosy cases. Lepr. India 27(1955)149-152.
12. GILL, H. K., MUSTAFA. A. S. and GODAL, T. Vaccination of human volunteers with heat killed M. leprae: local responses in relation to the interpretation of the lepromin reaction. Int. J. Lepr. 56(1988)36-44.
13. GRANGE, J. M. Environmental mycobacteria and human disease. Lepr. Rev. 62(1991)353-361.
14. GROENEN, G., PATTYN, S. R., GHYS, P., TSHILUMBA, K., KUYKENS, L. and COLSTON, M. J. A longitudinal study of the incidence of leprosy in a hyperendemic area in Zaire, with special reference to PGL-antibody results. Int. J. Lepr. 58(1990)641-650.
15. ILANGUMARAN, S., SHANKARNARAYAN, N., RAMU, G. and MUTHUKKARUPPAN, V. Antibody response to recombinant 65-kDa, 70-kDa and 18kDa mycobacterial antigens in leprosy patients and healthy contacts in a leprosy endemic population. Int. J. Lepr. 62(1994)245-255.
16. ILANGUMARAN, S., SHANKAR NARAYAN, N., RAMU, G. and MUTHUKKARUPPAN, V. R. Cellular and humoral responses to recombinant 65-kD antigen of Mycobacterium leprae in leprosy patients and healthy controls. Clin. Exp. Immunol. 96(1994)79-85.
17. KANCHANA, M. V., LAKSHMINARAYANA, C. S., SENGUPTA, U., SINHA, S. and RAMU, G. An appraisal of enzyme linked immunosorbent assay (ELISA) and serum antibody competition test (SACT) in leprosy. Indian J. Lepr. 64(1992)42-50.
18. KAPLAN, G. and COHN, Z. A. Leprosy and cell mediated immunity. Curr. Opin. Immunol. 3(1991)91-96.
19. NOORDEEN, S. K. The epidemiology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 15-30.
20. NOORDEEN, S. K. A look at world leprosy. Lepr. Rev. 62(1991)72-86.
21. RAMU, G. Genesis of leprosy lesions. Indian J. Lepr. 59(1987)133-144.
22. RIDLEY, D. S. and JOPLING, W . H. Classification of leprosy according to immunity-a five-group system. Int. J. Lepr. 34(1966)255-273.
23. RIDLEY, D. S. and JOB, C. K. The pathology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 100-133.
24. SALGAME. P., MODLIN, R. L. and BLOOM, B. R. On the mechanism of human T cell suppression. Int. Immunol. 1(1989)121-129.
25. SAMPAIO, E. P., MOREIRA, A. L., KAPLAN, G., ALVIM, M. F. S., DRUPPE, N. C, MIRANDA, C. F. and SARNO, E. N. Mycobacterium leprae induced interferon-gamma production by household contacts of leprosy patients: association with the development of active disease. J. Infect. Dis. 164(1991)990-993.
26. SENGUPTA, U. Does serology help in diagnosing early leprosy? Indian J. Lepr. 65(1993)39-48.
27. SENGUPTA. U., SINHA, S., RAMU, G.. LAMB, J. and IVANYI, J. Suppression of delayed hypersensitivity reactions to tuberculin by M. leprae antigens in patients with lepromatous and tuberculoid leprosy. Clin. Exp. Immunol. 68(1987)58-64.
28. SHEELA, R., SHANKER NARAYAN, N., RAMU, G. and MUTHUKKARUPPAN, V. R. IgG subclass antibodies to mycobacterial sonicate and recombinant antigens in leprosy. Lepr. Rev. 66(1994)10-18.
29. SHEPARD, C. C. Immunity to leprosy and the Mitsuda reaction. Int. J. Lepr. 52(1984)74-77.
30. SINHA, S., MCENTEGART, A., GRIDHAR, B. K., BHATIA, A. S. and SENGUPTA, U. Appraisal of two Mycobacterium leprae-spcciUc serological assays for monitoring chemotherapy in lepromatous (LL/BL) leprosy patients. Int. J. Lepr. 57(1989)24-32.
31. SMITH, P. G. The serodiagnosis of leprosy. Lepr. Rev. 63(1992)97-100.
32. ULRICH, M., SMITH, P. G., SAMPSON, C., ZUNIGA, M., CENTENO, M., GARSIA, V., MANRIQUE, X., SAGALDO, A. and CONVITT, J. IgM antibodies to native phenolic glycolipid I in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of the risk of leprosy. Int. J. Lepr. 59(1991)405-415.
33. WATSON, J. D. Prospects for new generation vaccines for leprosy: progress, barriers and future prospects. Int. J. Lepr. 57(1989)834-843.
34. YAMAMURA, M., UYEMURA, K., DEANS, R. J., WEINBERG, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy skin lesions. Science 254(1991)277-279.
35. YOUNG, D. B. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. Ph.D., Department of Immunology, School of Biological Sciences, Madurai Kamaraj University, Madurai 625021, India.
2. Ph.D., Department of Immunology, School of Biological Sciences, Madurai Kamaraj University, Madurai 625021, India.
3. Ph.D., Department of Immunology, School of Biological Sciences, Madurai Kamaraj University, Madurai 625021, India.
4. M.B.B.S., D.T.M.&H., Voluntary Health Services, Leprosy Project, Sakthinagar 638315, India.
5. M.D., Voluntary Health Services, Leprosy Project, Sakthinagar 638315, India.
Reprint requests to Dr. S. llangumaran at his present address: Department of Pathology, CMU, 1 rue Michel Servet, 1211 Geneva 4, Switzerland.
Received for publication on 1 August 1995.
Accepted for publication in revised form on 6 November 1995.