- Volume 64 , Number 1
- Page: 26–36
Immunotherapy of far-advanced lepromatous leprosy patients with low-dose convit vaccine along with multidrug therapy (Calcutta trial)
ABSTRACT
This report describes a promising mode of treatment of lepromin-unresponsive, faradvanced, lepromatous (LL) leprosy patients with antileprosy vaccines as an adjunct to multidrug therapy (M DT). The Trial Groups included 50 highly bacilliferous, lepromin-negative, untreated LL patients. They were given MDT for 2 years. Of them, 30 patients were administered a mixed antileprosy vaccine containing killed Mycobacterium leprae of human origin plus A/. bovis BCG. The remaining 20 patients were given M. bovis BCG. Depending on the severity of lepromin unresponsiveness, they were given one to six inoculations at 3-month intervals. Another 20 similar LL patients were taken in the Control Group. They were given only MDT for 2 years. F rom the start of the study, all patients belonging to the Trial and Control Groups were followed every 3 months for clinical, bacteriological and immunological outcomes. Within 2 years all 50 patients of the Trial Groups and 19 of the 20 patients of the Control Group became clinically inactive and bacteriologically negative. However, the clinical cure and the falls of the bacterial and morphological indexes were much faster in those patients receiving the mixed vaccine therapy than in those patients who were given BCG plus MDT or only MDT. The immunological improvements in the patients of the Trial and Control Groups were assessed by: a) lepromin testing at the beginning of the study and at 3-month intervals and also by b) the in vitro leukocyte migration inhibition (LMI) test at both the beginning and end of the study. As the patients were given more and more vaccinations, the incidence of lepromin conversion increased, more so in the patients receiving the mixed vaccine. Thus, 63%, 15% and 5% of the patients became lepromin positive in those patients receiving the mixed vaccine, BCG, and MDT only, respectively. Lamentably, the vaccine-induced lepromin positivity was temporary and faded away within several months. At the beginning of the study, the LMI test against specific M. leprae antigen was negative in all patients of both the Trial and Control Groups. After the end of the chemo-immunotherapy schedule, the LMI test became positive in 50% and 20% of LL patients receiving the mixed vaccine and BCG, respectively. None of the Control Group could show LMI positivity after completion of the MDT schedule. These results show that treatment of LL patients with the mixed vaccine and MDT could quickly reverse the clinical course of the disease, remove immunologic anergy in some patients, and induce a rapid decrease in the bacterial load in them.RÉSUMÉ
Ce rapport décrit un mode de traitement prometteur par vaccins anti-lépreux comme complément à la polychimiothérapic (PCT) pour des malades de la lèpre présentant une lèpre lépromateuse très avancée (LL) ne répondant pas à la lépromine. Le groupe expérimental comprenait 50 patients LL non traités, hautement bacillifères et négatifs à la lépromine. On leur a donné la PCT pendant deux ans. Parmi eux, 30 ont reçu le vaccin anti-lèpre mixte, contenant du Mycobacterium leprae d'origine humaine tué par la chaleur; les autres 20 patients ont reçu du BCG de M. bovis. Selon le degré de non-réponse à la lépromine, ils ont reçu de une à six injections à trois mois d'intervalle.Un autre groupe de 20 patients LL semblables ont été pris dans le groupe témoin. Ils ont reçu seulement la PCT pour deux ans.
Depuis le début de l'étude, tous les patients appartenant au groupe expérimental et au groupe témoin ont été suivis tous les trois mois du point de vue clinique, bactériologique et immunologiquc. Endéans deux ans, tous les 50 patients du groupe expérimental et 19 des 20 témoins du groupe témoin sont devenus cliniquement inactifs et bactériologiquement négatifs. Cependant, la guérison clinique et la chute des indices bactériens et morphologiques furent beaucoup plus rapides parmi les patients ayant reçu un vaccin mixte que parmi les patients ayant reçu le BCG avec la PCT ou la PCT seule. Les améliorations immunologiqucs chez les patients du groupe expérimental et du groupe témoin ont été évaluées par a) le test à la léprominc au début de l'étude et à chaque intervalle de trois mois, et également par b) le test d'inhibition de la migration leucocytaire (IML) au début et à la fin de l'étude. Comme les patients recevaient de plus en plus de vaccins, l'incidence de la conversion à la léprominc augmentait, plus encore parmi les patients recevant le vaccin mixte. Ainsi, respectivement 63%, 15% et 5% des patients devinrent positifs à la léprominc parmi les patients recevant le vaccin mixte, le BCG, et la PCT seule. Malheureusement, la positivité à la léprominc induite par le vaccin ne fut que temporaire et disparut en l'espace de quelques mois. Au début de l'étude, le test IML vis-à-vis de l'antigène spécifique de M. leprae était négatif pour tous les patients des groupes expérimental et témoin. A la fin du schéma de chimio-immunothérapic, le test IML devint positif chez respectivement 50% et 20% des patients LL recevant le vaccin mixte et ceux recevant le BCG. Aucun patient du groupe témoin n'a pu montrer une positivité à l'IML à la lin du schéma PCT.
Les résultats montrent que le traitement des patients LL avec un vaccin mixte et la PCT pourrait modifier rapidement l'évolution clinique de la maladie, supprimer l'anergie immunologique chez certains patients, et induire une diminution rapide de la charge bactérienne.
RESUMEN
Este reporte describe un modo promisorio de tratamiento con vacunas antileprosas complementarias al tratamiento con poliquimioterapia (PQT) de pacientes con lepra lepromatosa avanzada (LL) negativos a la lepromina. El grupo de prueba incluyó 50 pacientes LL altamente bacilíferos, lepromina negativos y sin tratamiento previo. Los pacientes recibieron PQT durante 2 años. De ellos, 30 pacientes recibieron una vacuna mixta preparada con Mycobacterium leprae inactivado, de origen humano; los 20 pacientes restantes recibieron M. bovis. BCG. Dependiendo de la severidad de la anergia a la lepromina los pacientes recibieron de una a seis dosis de vacuna con intervalos de 3 meses. Otros 20 pacientes LL similares, recibieron sólo la PQT durante 2 años (grupo control).Desde el comienzo del estudio, todos los pacientes fueron valorados cada 3 meses desde el punto de vista clínico, bacteriológico c inmunológico. En 2 años todos los pacientes tratados con vacunas y 19 de los 20 pacientes del grupo control se tornaron clínicamente inactivos y bacteriológicamente negativos. Sin embargo, la curación clínica y la caída de los índices bacteriológico y morfológico fueron mucho más rápidas en los pacientes que recibieron la terapia con vacuna mixta que en los pacientes que recibieron BCG más PQT o solo la PQT. La mejoría inmunológica en los pacientes de los grupos de ensayo y control se estableció (a) por pruebas dérmicas con lepromina al inicio del estudio y después a intervalos de 3 meses, y (b) por la prueba de inhibición de la migración de leucocitos (IML) realizada al inicio y al final del estudio. La frecuencia de conversión positiva a la lepromina aumentó conforme los pacientes recibieron más y más vacunaciones, y esto fue más claro en los pacientes que recibieron la vacuna mixta. Así, el 63%, cl 15%, y el 5% de los pacientes se tornaron lepromino-positivos cuando recibieron, respectivamente, la vacuna mixta, BCG, y la PQT sola. Lamentablemente, la positividad a la lepromina inducida por la vacunación fue temporal y se desvaneció por completo después de varios meses. Al comienzo del estudio, la prueba de IML con antígenos especificos de M. leprae fue negativa en todos los pacientes de ambos grupos. Al final de la PQT, la prueba de IML se hizo positiva en el 50% y el 20% de los pacientes LL que recibieron la vacuna mixta o BCG, respectivamente. Ninguno de los pacientes del grupo control mostró una prueba de IML positiva después de completar el tratamiento con PQT.
Estos resultados muestran que el tratamiento de los pacientes LL con vacuna mixta y PQT puede revertir rápidamente el curso clínico de la enfermedad, remover la anergia inmunológica en algunos pacientes, e inducir una rápida disminución de la carga bacteriana en ellos.
Several claims had been made in recent years on the importance of immunotherapy of lepromin-negative leprosy patients in conferring resistance to relapse or re-infection in them (8). Perhaps one of the most important aspects of these studies was that following immunization with an antileprosy vaccine, cell-mediated immunity (CMI) against Mycobacterium leprae could be mounted in a significant number of previously lepromin-unresponsive patients with the most progressive form of leprosy. They inoculated their patients with 8 to 10 doses of antileprosy vaccines containing 6.4 x 108 killed M. leprae (A) and 0.1 mg BCG (5). Recently, we administered heat-killed M. leprae of human origin, BCG, or a mixture of the two into healthy but high-risk household contacts of multibacillary (MB) leprosy patients to protect them against the development of clinical leprosy, and this intervention reduced the incidence of illness from 13% to 7% (4).
The present study on the immunotherapy of leprosy patients by antileprosy vaccines was an extension of our earlier work on immunoprophylaxis against leprosy by employing similar vaccines. Our aim was to determine the efficiency of the two currently popular antileprosy vaccines in the treatment of fresh, lepromin-unresponsive, faradvanced, lepromatous (LL) patients in terms of clinical recovery, early reduction of bacterial load and augmentation of impaired CMI. The numbers of heat-killed M. leprae and BCG per dose in our mixed vaccine were only 1.6 x 107 and 1.5 x 105, respectively, much less than that employed by Convit and associates (5). The lower doses of these antigens were used in an effort to minimize adverse reactions.
MATERIALS AND METHODS
Human subjects
Seventy previously untreated, polar lepromatous (LL) leprosy patients (58 males and 12 females) were taken from the Outpatient Department of the School of Tropical Medicine, Calcutta, India. The diagnosis of leprosy was based on clinical, bacteriological and histological findings as well as lepromin testing. Slit-skin smear examination showed bacterial positivity in all cases. Their bacterial indexes (Bis) varied from 2.6 + to 4.0 + . They were all lepromin (Mitsuda reaction) negative.
Grouping of patients
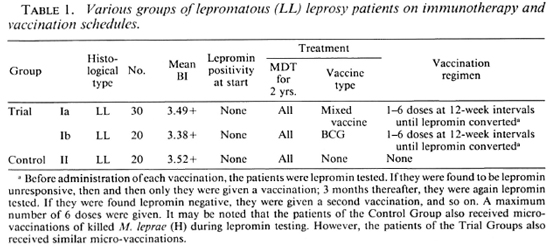
The 70 patients were divided randomly into two groups: Group I or Trial Group included 50 LL patients; Group II or Control Group included 20 LL patients. Group I was further divided into two subgroups: Subgroup la consisted of 30 LL patients (24 males and 6 females). Their mean age was 32.30 ± 9.95 (17-45) years. They were given a mixed vaccine along with MDT. Subgroup lb consisted of 20 LL patients (17 males and 3 females). Their mean age was 30.28 ± 7.93 (16-41) years. They were given BCG vaccination plus MDT. The remaining 20 LL patients (17 males and 3 females) were included in the Control Group (Group II). Their mean age was 35.55 ± 13.31 (21-70) years. They were administered only MDT. The mean durations of illness of the patients belonging to Groups la, lb and II were 4.0 ± 3.48, 3.24 ± 2.54, 4.93 ± 6.48 years, respectively. All cases were previously untreated. All patients were treated with MDT for 2 years.
Vaccines
(a) Each dose of the mixed vaccine contained 1.6 x 107 heat-killed M. leprae of human origin and 1.5 x 105 BCG (Japan) in 0.1 ml saline (4); (b) each dose of BCG (Japan) vaccine contained 1.5 x 105 bacteria in 0.1 ml saline. Before each inoculation all patients were tested with the standard lepromin (human origin) (routinely made in our laboratory). If they remained lepromin (Mitsuda reaction) unresponsive, only then were they given the next vaccination. The interval between two successive inoculations was 3 months. The number of vaccinations given in the trial patients varied from a minimum of one to a maximum of six, depending on the diration of their unresponsiveness to lepromin. The dose and schedule of vaccination is shown in Table 1.
Clinical outcome
After starting treatment, all of the patients were followed at 3-month intervals for 2 years to study their clinical course, any reversal reaction, and other complications.
Bacteriological negativity
The rates of reduction of the BI and morphological index (MI) were recorded at 3-month intervals by the standard slit-skin smear technique (2).
Immunological assessments
At the start of treatment, all patients were tested for lepromin reactivity and thereafter at 3-month intervals for 2 years. A suspension of autoclaved human-derived M. leprae (1.6 x 108/ml) was prepared in our laboratory from human leproma (ear); 0.1 ml of the suspension was injected intradcrmally at the flexor surface of the left arm near the elbow joint. Three to 4 weeks thereafter the late lepromin (Mitsuda) reaction was read. A palpable nodule of 3-mm diameter or more was taken as positive (10). The leukocyte migration inhibition (LMI) test was done against M. leprae antigen in all patients at the start and end of the study. Leukocyte-rich plasma samples were obtained from all patients and a cell suspension containing 4 x 106 cells in 1 ml of minimal essential medium (MEM) was used to fill capillary tubes. The tubes were incubated in migration chambers filled with a sonicated suspension of M. leprae (4). Areas of migration were determined by a camera lucida and the migration inhibition indexes were determined (1).
RESULTS
Clinical outcome
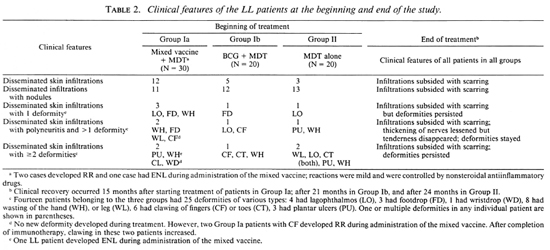
Table 2 shows the clinical features of the Trial and Control Groups at the beginning of the study. All 70 patients had disseminated dermal infiltrations. Within Group la, 11 patients had nodular lesions, and 7 had one or more deformities with nerve involvement and paralysis. Within Group lb, 12 patients had nodular lesions, and of them 3 had nerve involvement with one or more deformities. Within Group II, 13 patients had nodular lesions with 4 patients having one or more deformities. Clinical recovery was judged to be faster in the patients (Group la) receiving the mixed vaccine in comparison to Group lb patients receiving BCG, or Group II patients getting only MDT (Table 2). Skin infiltrations subsided with scarring. However, plantar ulcerations and other deformities, as well as paralysis, persisted. Nerve thickening and anesthesia lessened but nerve tenderness disappeared.
Deformities
Seven patients from Group la, 3 patients from Group lb and 4 patients from Group II had various deformities (Table 2). Of the 7 patients with deformities belonging to Group la (administered the mixed vaccine and MDT) 2 had disseminated skin infiltrations, polyneuritis and more than one deformity before starting immunotherapy. Two cases developed reversal reaction (RR) 7 to 8 months after starting treatment as evidenced by the appearance of erythematous plaques in their skin and pain in their limbs along with tenderness over the nerves. There was worsening of pre-existing clawing of both the hands and feet. The RR was controlled with nonsteroidal antiinflammatory drugs. No new nerve was involved during treatment.
Bis and Mis
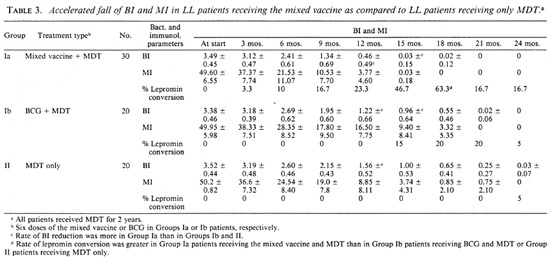
Although all patients of the Trial Groups and all but one of the Control Group became bacteriologically negative within the study period, the rates of fall of the BI and MI were faster in Group la patients receiving the mixed vaccine plus MDT than in Group lb patients receiving BCG plus MDT and Group II patients receiving MDT only (Table 3 and Fig. 1 a through f). During the first 6 months of the trial, the rates of fall of the BI and MI in patients receiving the mixed vaccine (Group la) were parallel with those for patients who received only MDT (Group II). During the next 6 months of the trial, however, the falls of the Bis and Mis of the patients in Group la were remarkably faster than those of the patients belonging to Groups lb and II. Thus, the mean BI of the patients in Group la declined to almost zero 15 months after starting the trial; it was 0.96+ and 1.00+ for the patients in Group lb and Group II, respectively, after the same period.
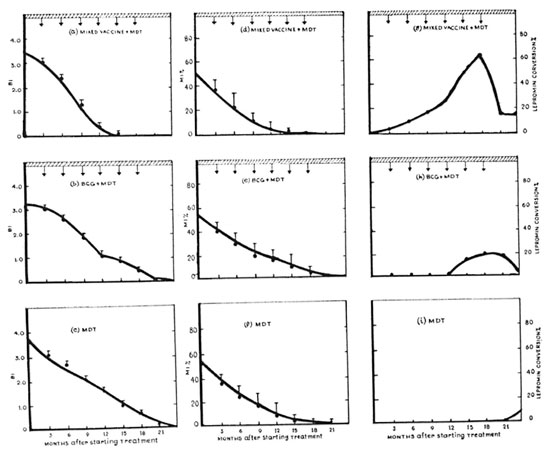
Fig. Ia. BI in 30 LL patients who received the mixed vaccine and MDT (Group la). III = MDT was givenfor 2 years; 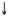 = mixed vaccine was given at 3-month intervals. Maximum number of inoculations given to thepatients was six.
= mixed vaccine was given at 3-month intervals. Maximum number of inoculations given to thepatients was six.
Fig. lb. Fall of BI in 20 LL patients who received BCG and MDT (Group lb).  = BCG was given at 3-month intervals. Maximum number of inoculations given to the patients was six.
= BCG was given at 3-month intervals. Maximum number of inoculations given to the patients was six.
Fig. lc. Fall of BI in 20 LL patients who received only MDT (Group II). Only one patient showed leprominpositivity 2 years after administration of MDT (see Fig. Ii).
Fig. Id. Rapid fall of MI among 30 patients who received the mixed vaccine and MDT (Group la).
Fig. le. Fall of MI among 20 patients who received BCG and MDT (Group Ib).
Fig. 1f Fall of Ml in patients who received only MDT (Group II).
Fig. lg. Rate of lepromin conversion in Group la after administration of the mixed vaccine + MDT.
Fig. Ih. Rate of lepromin conversion in LL patients (Group Ib) after administration of BCG vaccination + MDT.
Fig. Ii. Only one LL patient could show lepromin positivity after administration of MDT.
Immunological results
Lepromin conversion. Table 3 shows that with the increase in the number of inoculations there was a gradual increase of the rate of lepromin positivity among Group la patients (receiving the mixed vaccine plus MDT). Eighteen months after receiving multiple vaccinations, 63.3% of the patients showed lepromin positivity; the remaining 36.7% of the patients remained anergic to lepromin after receiving six inoculations. Figure la and lg illustrate that as the mean BI declined progressively, the rate of lepromin conversion among the patients rose gradually, indicating that a relationship might exist between these two parameters. However, although the bacteriological negativity in these patients persisted throughout the study period, lepromin positivity initiated by vaccination did not last long (Fig. lg). Figure lh shows that the incidence of lepromin positivity among Group lb patients was only 20% and the rest of the patients (80%) remained lepromin negative despite receiving six inoculations of BCG. In contrast, only one patient (5%) of the Control Group II (receiving only MDT) showed lepromin positivity and that occurred 2 years after starting chemotherapy (Fig. li). Disappointingly, the lepromin positivity initiated by the mixed vaccine and BCG faded away in Group la and Group lb patients after they stopped receiving vaccinations (Fig. 1g and h).
LMI conversion. Table 4 shows that before the start of treatment the LMI indexes for all patients of the Trial and Control Groups were above 80; hence the tests were negative.
Two years after the start of treatment, the mean LMI indexes in the patients from Groups Ia, Ib and II were 75.95, 86.4 and 93.35, respectively. The differences between the mean LMI indexes at the beginning and end of the study in Groups la and lb patients were statistically significant, while those in Group II patients were insignificant. Figure 2 indicates that 15 out of 30 Group la patients (50%) and 4 out of 20 Group lb patients (20%) showed LMI conversion. None of the 20 Group II patients showed LMI positivity despite the fact that they all (except one) became bactcriologically negative, and one showed lepromin positivity. These results indicate that the mixed vaccine containing killed M. leprae and BGG was a more efficient immunopotentiating agent than BCG alone.
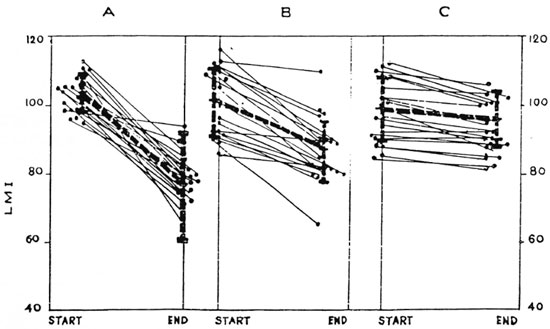
Fig. 2. Leukocyte migration inhibition (LMI) indexes against M. leprae sonicate in LL patients at thebeginning and end of 2 years of treatment. A = Group Ia, 30 LL patients receiving the mixed vaccine + MDT. B = Group lb, 20 LL patients receiving BCG + MDT. C = Group II, 20 LL patients receiving only MDT. Each dot represents one patient; thick interrupted lines = mean ± S.D. of LMI indexes. A LM1 index of 80 or below was taken as positive. No patient in the three groups showed LMI positivity at the beginning of treatment. After the end of treatment 15 patients of Group Ia, 4 patients of Group lb, and none of Group II could show LMI positivity
DISCUSSION
In 1982 Convit and associates treated 529 lepromin-nonrcactive leprosy patients with a vaccine containing a mixture of killed M. leprae (armadillo) and BCG and evaluated their clinical, bacteriological and immunological outcome (5). The rationale for using this vaccine combination was that when lepromin containing killed M. leprae was injected intradermally into Mitsuda-ncgative LL patients, a granuloma with undifferentiated macrophages full of bacilli was formed at the site of the injection. However, when a mixture of BCG and lepromin was injected intradermally, the bacilli were eliminated from the granuloma (7). Keeping this in mind, the present double-blind vaccine trial was conducted in Calcutta to study the value of treatment of far-advanced, lepromin-negativc, LL cases with a mixed vaccine containing BCG plus killed M. leprae (human) along with MDT (Table 1).
Modified mixed vaccine, its type, dose and efficacy
Instead of using armadillo-derived M. leprae, in this study we employed humanderived bacilli because it did not contain any animal proteins (9). Furthermore, in an attempt to avoid severe reversal reaction and nerve damage (such as lagophthalmos and footdrop) so often encountered during immunotherapy of borderline cases (3), we administered vaccines containing much fewer bacilli in comparison to that employed in the Venezuela trial. Convit, et al. gave their patients as many as eight to ten inoculations of a mixed vaccine containing 0.1 mg BCG supplemented by 6.4 x 108 killed M. leprae (A) (5). In the present trial we administered only six doses of a mixed vaccine containing only 1.5 x 105 M. hovis BCG and 1.6 x 107 killed Al. leprae (H). Even then, a quick reduction of the bacterial load within 18 months and a lepromin conversion as high as 63.3% were observed (Fig. 1). Only mild reactions occurred in 3 patients [RR in 2 and erythema nodosum leprosum (ENL) in 1] during the immunotherapy with the mixed vaccine (Table 2). All patients in our Trial Group (Groups la and lb) were Mitsuda-negative lepromatous patients. Perhaps immunotherapy with the mixed vaccine could not induce RR in these patients.
Clinical outcome
Immunotherapy accelerated clinical improvement in our patients, but failed to reduce deformities due to pre-existing nerve involvement. Nerve tenderness disappeared during the course of treatment. Clawing increased in two patients in Group la, perhaps due to mounting of CM1 and an immunologic struggle in the nerves causing nerve damage. This type of RR is invariably accompanied by mounting of CMI against M. leprae, resulting in the reduction of the bacterial population of active lesions. The RRs in our LL patients were mild. The absence of severe RR in our advanced polar LL patients during immunotherapy suggested that, on the one hand, the present mode of treatment was safe but, on the other hand, it indicated that it was very difficult to initiate immune augmentation in these patients with heavy bacillary loads. Perhaps their immunologic barrier could have been overcome to some extent by increasing the number of inoculations and the quantum of antigens of the mixed vaccine, but such practice has its own hazards and limitations. Multiple injections of BCG might stimulate Th2 lymphocytes, leading to the release of interleukin 4 (IL-4) and suppression of Th1 lymphocytes and CMI (14,17). A similar finding was reported by Shannon, et al., who employed an animal model. They could induce RR in M. leprae -infected, athymic nude mice following adoptive transfer of splenic-cell suspensions derived from nonimmunized nu/+ mice and from those vaccinated with heat-killed M. leprae, BCG, or a mixture of both (16).
Accelerated reduction of Bis and Mis and lepromin conversion
The rapid fall of the BI and the rise of the lepromin conversion rate in Group la patients suggested that the mixed vaccination could gradually build up the M. leprae -specific immune response 6 to 12 months after starting immunotherapy in about two-thirds of our patients. This presumably led to activation of their macrophages loaded with intracellular M. leprae, release of lethal hydroxyl radicals in an iron-dependent reaction with the superoxide anion, and elimination of bacteria (12). But this notion of a relationship between mounting of the CMI and bacterial clearance failed to offer an explanation for the observed persistent lepromin anergy among the remaining onethird of the patient population after receiving six inoculations of the mixed vaccine, despite their Bis becoming zero (Table 3). This indicated that the accelerated fall of the BI also might occur in some LL patients who remained persistently lepromin negative.
Immune augmentation in LL patients following administration of mixed vaccine
In the absence of a test that truly measures protective immunity against leprosy, we employed Mitsuda and LMI test positivities as parameters to gauge immunologic potentiation. Mitsuda positivity, the hallmark of protective immunity against leprosy, could be induced in 63% of our LL patients receiving the mixed vaccine. But, unlike natural Mitsuda positivity which remains life long, the vaccine-driven Mitsuda reactivity was not permanent.
A positive LMI test suggested the ability of M. leprae -specihe T cells to release lymphokines when challenged with specific antigen (5). All patients of the Trial and Control Groups were LMI negative at the start of the study, but 50% of the LL patients receiving the mixed vaccine, 20% of the patients receiving BCG, and none of the LL patients receiving only MDT showed LMI positivity (Table 4). The mean LMI index (75.95) was less in those patients receiving the mixed vaccine + MDT than that (86.4) observed in the patients receiving BCG + MDT (Table 4). Thus, by LMI immune augmentation with the mixed vaccination was more elfective than that oifercd by BCG vaccination.
Rada, et at. recently administered 2 to 10 doses of a mixture of heat-killed M. leprae (6 x 108 bacilli) and 0.2 mg of live BCG in MB patients in a period of 1 to 3 years. They found that the decrease in the BI was proportional to the increase of the lymphocyte transformation positivity test (LTT) to a soluble extract of Al. leprae. Lepromin positivity in these MB patients was 5% initially, which increased to 75% at the end of immunotherapy, while 34% of the LL patients showed LTT conversion (15).
Lepromin negativity persists in some LL patients following administration of mixed vaccine
Table 3 shows that an increasing proportion of patients underwent lepromin conversion after successive inoculations of the mixed vaccine. Thus, even within one histologic type of leprosy (LL) there apparently were variations of the grades of lepromin unresponsiveness, so much so that it was impossible to initiate typical lepromin granuloma (4) in some LL patients with the multiple vaccinations.
Mixed vaccine vs. BCG employed in immunotherapy and immunoprophylaxis
According to Harboc, it is mandatory to compare the effect of any leprosy vaccine with the effect of BCG alone on a suitable control group within the trial area at the same time (10). Convit, et al. (6) and later Chaudhury and associates (4) reported that the addition of killed M. leprae to BCG could not improve the efficacy of BCG for protecting high-risk contacts against the development of leprosy. On the other hand, the results of the present study on immunotherapy show that the immunotherapy of LL patients with a mixed vaccine was superior to BCG.
Mustafa, et al. have recently claimed that peripheral blood mononuclear cells from individuals immunized with M. leprae and BCG responded to the M. leprae 18-kDa and 65-kDa heat-shock protein of M. bovis BCG (13). It is yet to be established how long the changes in reactivity induced in our patients by administration of the mixed vaccine would last and to what extent they were related to the increased ability to limit the multiplication of M. leprae in the LL patients (10). A multicenter, long-term, followup study is, thus, warranted because immunotherapy with a mixed vaccine could become a part of future MDT programs, at least for highly bacillated LL patients.
REFERENCES
1. BLOOM, B. R. and GLADE, P. R. In VitroMethods in Cell-MediatedImmunity. New York: Académie Press, 1971.
2. BRYCESON, A. and PFALTZGRAFF, R. E. Leprosy. 2nd cdn. Edinburgh: Churchill Livingstonc, 1979, pp. 28-64.
3. CHAUDHURY, S., FATEDAR, A. and TALWAR, G. P. Conversion in repeated lepromin négative BL/LL patients after immunization with autoclaved Myœbacterium w . Int. J. Lepr. 51(1982)159-165.
4. CHAUDHURY, S., HASRA, S. K., SAHA, B., MAZUMDER, B., CHATTOPADHYA, D. and SAHA, K. An eight-ycar ficld trial on antileprosy vaccines among high-risk household contacts in the Calcutta metropolis. Int. J. Lepr. 62(1994)389-394.
5. CONVIT, J., ARANZAZU, N., ULRICH, M., PINARDI, M. E., REYES, 0. and ALVARADO, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and Mitsuda-negative contacts. Int. J. Lepr. 50(1982)415-420.
6. CONVIT, J., SAMPSON, C, ZUFIIJA, M., SMITH, P. G., PLATA, J., SILVA, J., MOLINA, J., PINARDI, M. E., BLOOM, B. R. and SALGADO, A. Immuno-prophylactic trial with combined Mycobacterium lep- rac/BCG vaccine against leprosy: preliminary results. Lancet 339(1992)446-450.
7. CONVIT, J., ULRICH, M. and ARANZAZU, N. Vaccination in leprosy-observations and interpretations. Int. J. Lepr. 48(1980) 62-65.
8. CONVIT, J., ZUFIUA, M., ULRICH, M., ARANZAZU, N. and PINARDI, M. E. Immunotherapy and immunoprophylaxis of leprosy. In: Leprosy. Chatterjec, B. R., cd. Jhalda, India: Leprosy Field Research Unit, 1993, pp. 536-546.
9. DYACHINA, M. N., PYNCHUCK, L. M., LAZOVSKAYA, A.L. and JUSCENKO, A. A. Assessment of the purity of M. leprae preparations from tissues of leprosy-infected laboratory animals. Int. J. Lepr. 62(1994)299-301.
10. HARBOE, M. The immunology of leprosy. In: Leprosy. Hastings, R. C, cd. Edinburgh: Churchill Livingstone, 1985, pp. 53-87.
11. JAMET, P. and Ji, B. (Marchoux Chemotherapy Study Group). Relapses in multibacillary leprosy patients after stopping treatment with rifampincontaining combined regimen. Int. J. Lepr. 60(1992)525-535.
12. KLEIN, J. Defence reactions mediated by phagocytes. In: Immunology. Oxford: Blackwell Scientific Publications, 1992, pp. 31 1-335.
13. MUSTAFA, A., LUNDIN, K. E. A. and OFTUNG, F. Human T-cells recognize mycobacterial heat shock proteins in the context of multiple HLA-DR molecules-studies with healthy subjects vaccinated with Mycobacterium bovis BCG and Mycobacterium leprae. Infect. Immun. 61(1993)5294-5301.
14. OTTENHOFF, T. H. M. Immunology of leprosy: lessons from and for leprosy. (Editorial) Int. J. Lepr. 62(1994)108-121.
15. RADA, E., ULRICH, M., ARANZAZU, N., SANTAELLA, C, GALLINOTO, M., CENTENO, M., RODRIGUEZ, V. and CONVIT, J. A longitudinal study of immunologic reactivity in leprosy patients with immunotherapy. Int. J. Lepr. 62(1994)522-558.
16. SHANNON, E. J., CHEHL, S., JOB, C. K. and HASTINGS, R. G. Adoptively transferred reactivity to M. leprae in nude mice infected with M. leprae. Clin. Exp. Immunol. 70(1987)143-151.
17. SIELING, P. A., ABRAMS, J. S . and YAMAMURA, M. Immunosuppressive roles of IL-10 and IL-4 in human infection; in vitro modulation of T-cell response in leprosy. J. Immunol. 150(1993)5501-5510.
1. M.B.B.S., D.D.V., Demonstrator in Leprology.
2. M.D., D.C.P., Ph.D., Professor of Pathology and Director (retired).
3. M.B.B.S., D.C.P., D.T.M.&H., Ph.D., Reader in Leprology (retired).
4. M.B.B.S., D.T.M.&H., Lecturer in Leprology, School of Tropical Medicine, Calcutta, India.
5. M.B.B.S., M.Sc, Ph.D. (Penn., U.S.A.), Professorof Immunology (retired), Delhi University, Delhi, India.
Reprint requests to Dr. Kunal Saha, 45A Sova Bazar Street, Calcutta 700 005, India.
Received for publication on 19 June 1995;
Accepted for publication in revised form on 6 November 1995.