- Volume 64 , Number 1
- Page: 37–43
Reduction of epidermal cell proliferation in skin lesions in lepromatous leprosy is greater than in indeterminate and tuberculoid leprosy lesions
ABSTRACT
We have compared epidermal cell proliferation in skin biopsies f rom areas with lesions to contralateral areas without lesions in patients with indeterminate, tuberculoid and lepromatous leprosy. Cell proliferation was determined as the percentage of labeled cells in the basal and suprabasal epidermal layers, using autoradiographic preparations of skin biopsies taken 1 hr after a 3H-thymidine intradermal injection. We have found a significant reduction in epidermal cell proliferation in areas with lesions in the three groups of patients. The greatest reduction occurred in lepromatous patients. In lesions of patients with indeterminate or tuberculoid leprosy, the reduction was the same, and in both groups it was smaller than in lepromatous patients. In the areas without lesions, the index of labeled cells was similar to that of "normal" skin of nonleprosy patients.In the contralateral unaffected areas f rom leprosy patients and in "normal" skin f rom nonleprosy patients, as well as in affected areas f rom patients with indeterminate leprosy, epidermal cell labeling was greater in the suprabasal layer than in the basal layer.
In lesions of lepromatous patients, cell labeling was greater in the basal layer than in the suprababal layer. Our findings suggest that the reduction of epidermal cell proliferation in leprosy patients is restricted to the cell-mediated immune response, more intense in lepromatous leprosy. It does not seem to be related to denervation, which is greater in tuberculoid leprosy.
RÉSUMÉ
Nous avons comparé la prolifération de cellules épidermiques dans des biopsies cutanées provenant de régions présentant des lésions avec celles provenant de régions contralatérales sans lésions chez des patients présentant une lèpre indéterminée, tuberculoide et lépromateuse. La prolifération cellulaire a été déterminée par le pourcentage de cellules marquées dans les couches épidermiques básales et suprabasales, utilisant des préparations autoragiographiques de biopsies cutanées prélevées une heure après l'injection intradermique de 3H-thymidinc. Dans les trois groupes de patients, nous avons trouvé une réduction significative de la prolifération cellulaire épidermique dans les zones présentant des lésions. La réduction la plus forte survenait chez les patients lépromateux. Au niveau des lésions de patients présentant une lèpre indéterminée ou tuberculoide, la réduction était semblable, et dans les deux groupes elle était plus faible que chez les malades lépromateux. Dans les régions sans lésions, le pourcentage de cellules marquées était similaire à celui de la peau "normale" de témoins non-lépreux.Dans les régions contralatérales non atteintes de malades de la lèpre et dans la peau "normale" de témoins non-lépreux, ainsi que dans les régions atteintes de patients présentant une lèpre indéterminée, le pourcentage de cellules épidermiques marquées était plus élevé dans la couche suprabasale que dans la couche basale. Dans les lésions des malades lépromaleux, le pourcentage de cellules marquées était plus élevé dans la couche basale que dans la couche suprabasale.
Nos observations suggèrent que la réduction de la prolifération cellulaire épidermique chez les malades de la lèpre est limitée à la réponse immunitaire à médiation cellulaire, étant plus forte dans la lèpre multibacillaire. Cela ne semble pas être associé à une dénervation, qui est plus importante dans la lèpre tuberculide.
RESUMEN
Se comparó la proliferación de las células epidérmicas en las biopsias de piel de áreas con lesiones con aquellas de regiones contralatcrales sin lesiones, en pacientes con lepra indeterminada, tuberculoide y lepromatosa. La proliferación celular se determinó como el porcentaje de células marcadas en las capas epidérmicas basai y suprabasal utilizando preparaciones autorradiográñeas de biopsias de piel tomadas 1 h después de la inyección intradérmica de timidina tritiada.Se encontró una significante reducción en la proliferación de células epidérmicas en las áreas con lesiones de los 3 grupos de pacientes. La mayor reducción ocurrió en los pacientes lepromatosos. En las lesiones de los pacientes con lepra indeterminada o tuberculoide, la reducción fue la misma, y en ambos grupos fue más pequeña que en los pacientes lepromatosos. En las áreas sin lesiones, el índice de células marcadas fue similar al de la piel normal de los pacientes no leprosos.
En las áreas contralatcrales no afectadas de los pacientes con lepra y en la piel normal de los individuos sin lepra, así como en las áreas afectadas de los pacientes con lepra indeterminada, el mareaje de las células epidérmicas fue mayor en la capa suprabasal que en la capa basai. En las lesiones de los pacientes lepromatosos el mareaje de células fue mayor en la capa basal que en la suprabasal.
Nuestros hallazgos sugieren que la reducción de la proliferación de las células epidérmicas en los pacientes con lepra (más intensa en la lepra lepromatosa) está relacionada con el nivel de respuesta inmune celular y que no parece estar relacionada con denervación, la cual es mayor en la lepra tuberculoide.
Leprosy, an infectious disease, shows a continuous spectrum of clinical manifestations between two poles -tuberculoid and lepromatous. Ridley and Jopling (19) combined clinical manifestations and histopathologic changes, and described a livegroup classification system covering the whole spectrum: tuberculoid (TT), borderline tuberculoid (BT), borderline (BB), borderline lepromatous (BL), and lepromatous (LL). It is well known that it is the cellmediated immune response that determines the specific clinical appearance of this disease (4). At the tuberculoid pole, skin lesions show a small number of bacilli, activated macrophages, and a high ratio of T-helper, T-cytotoxic/T-suppressor, and interleukin 1 + (IL-1 +) cells. The granulomatous infiltrate has an epithelioid character. Toward the lepromatous pole, T-helper and T-cytotoxic cells decline, while T-suppressor and IL-1+ cells increase in number, macrophages are heavily infected, and their activation decreases (22). Langerhans' cells and the number of keratinocytes as well as Ia+ phenotype expression by keratinocytes also correlate with the clinical and morphologic spectrum of leprosy (9).
A dermal inflammatory process with an early and preferential impairment of skin nerves is one of the unique peculiarities of leprosy. Skin areas with lesions are anesthetic. The dermal inflammatory process and changes in the nerves have been widely studied (6,15). However, epidermal changes rarely have been studied (9,13,18). Trophic ulcers are one of the most important clinical features of leprosy. Although it has been suggested that they might be caused by nerve impairment, which leads to cutaneous anesthesia, a decreased epidermal proliferation could also contribute to their cause. Recently we have shown that epidermal cell proliferation is reduced in the skin lesions of leprosy patients (14). We now show that epidermal cell proliferation is significantly reduced in indeterminate, TT, and LL patients. The smallest reduction occurs in indeterminate leprosy, and the highest in lepromatous leprosy.
MATERIALS AND METHODS
Seventeen leprosy patients (40 to 60 years old, but 1 of whom was 10 years old) both males and females, newly diagnosed and untreated, and classified as indeterminate, TT, and LL were included in this study. After informed consent was obtained, each patient received a 0.1-ml 3H-thymidine 100 / µ Ci (Sp. Act. 6.7 Ci/mol; Dupont, Boston, Massachusetts, U.S.A.) local intradermal injection at a site showing a cutaneous anesthetic lesion and at a contralateral apparently unaffected site. Injection sites were either on the trunk or the limbs. One hr later, 4.0-mm punch biopsies were made at both injection sites. The entire procedure always was performed during the morning. The biopsy fragments were fixed in 10% formaldehyde containing 10% glycerine for 24 hr. Paraffin sections (4- µm thick) were taken either for autoradiography or for staining with hematoxylin and eosin (H&E) or with Fite-Faraco stain. The histopathologic classification of the patients was establisheci according to Ridley and Jopling (19). In five other patients, the clinical diagnosis of leprosy was not confirmed by histopathologic examination, and they were found to have nonspecific dermatitis. The microscopic findings being inconspicuous, these biopsies were considered to be from "normal" skin, and were used as nonleprosy controls.
For autoradiography, every fifth section was taken and covered with Kodak AR10 stripping film, according to Pelc (16), exposed for 28 days at 4ºC, developed and stained with H&E. The percentages of basal and suprabasal labeled cells were determined as the ratio between the number of basal or suprabasal labeled cells and the total number of basal cells counted. The total number of cells counted varied between 2600 and 26,000 cells/biopsy. Cells with at least five silver grains over the nucleus were considered to be labeled (26).
For statistical analysis the Mann-Whitney U test and the Kruskal-Wallis test with a level of significance set at 5% were used.
RESULTS
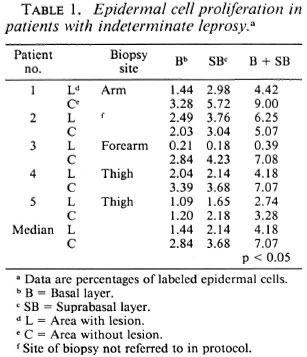
Indeterminate leprosy. The epidermal cell proliferation in biopsies from live patients was determined. A significant reduction (p < 0.05) in the percentage of labeled cells in the areas with lesions (median 4.18) as compared to the contralateral areas without lesions (median 7.07) was found. In only one patient (No. 2) was labeling in the area with lesions greater than in the contralateral area. Cell labeling in the suprabasal layer was greater than in the basal layer in all biopsies, both in areas with and without lesions, but in patient No. 3 the area with lesions had cell labeling slightly greater in the basal layer than in the suprabasal layer (Table 1).
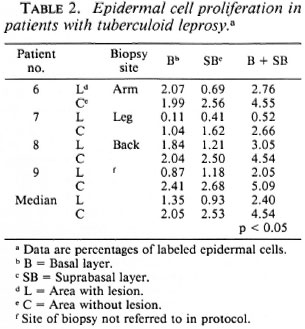
Tuberculoid leprosy (TT and BT). In all four patients epidermal cell labeling in areas with lesions (median 2.40) as compared to contralateral unaffected areas (median 4.54) was significantly reduced (p < 0.05). Cell labeling in the suprabasal layer was greater than in the basal layer in all four biopsies from unaffected areas, as well as in areas with lesions from two patients (Nos. 6 and 8; Table 2).
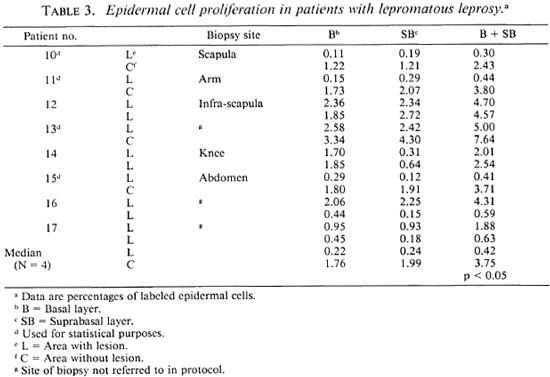
Lepromatous leprosy (LL and BL). In this group, eight patients were studied. In 4 of them (Nos. 12, 14, 16, 17) the contralateral biopsies, which clinically appeared to be from unaffected areas, at microscopic examination showed lesions. In two of these patients (Nos. 12 and 14) both biopsies showed rather similar labeling indexes; in the other two (Nos. 16 and 17) large differences were found. For statistical analysis, only the four patients from whom biopsies with and without microscopic lesions were available were used. In these four patients (Nos. 10, 11, 13, 15) epidermal cell proliferation in areas with lesions (median 0.42) as compared to contralateral unaffected areas (median 3.75) was significantly reduced (p < 0.05). Labeling in areas with lesions was lower than in unaffected areas in all four patients. In 3 out of 4 biopsies from areas without lesions, cell labeling in the suprabasal layer was greater than in the basal layer. In 8 out of 12 biopsies from areas with histologic lesions, cell labeling in the basal layer was greater than in the suprabasal layer (Table 3).
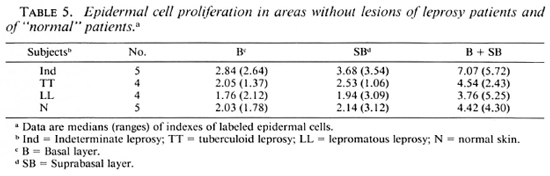
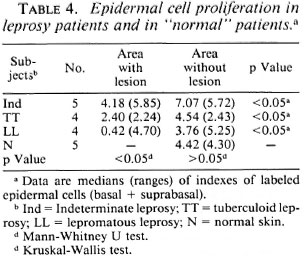
"Normal" skin. Epidermal cell proliferation in biopsies from contralateral areas without lesions of patients from all three groups was similar. There also were no differences in epidermal cell proliferation between these biopsies and those from "normal," nonleprous controls (Tables 4 and 5). In the latter, cell proliferation was also greater in the suprabasal layer than in the basal layer (Table 5).
DISCUSSION
The results presented here show that in leprosy patients epidermal cell proliferation in areas of the skin with lesions as compared to contralateral unaffected areas is significantly reduced. Thus, epidermal cell proliferation in cutaneous lesions of leprosy patients differs from other chronic inflammatory pathologies of the skin in which epidermal cell proliferation is increased (9,20,26).
Cutaneous neuritis, one of the peculiar features of leprosy, leads to a progressive deterioration in cutaneous sensitivity in the corresponding area (15). There is some evidence that cutaneous nerves might influence epidermal cell proliferation. Superficial nerves, Schwann cells, and perineural cells are present in increased numbers in psoriatic skin (24). A reduction in the mitotic index of dermal cells after sectioning of anterior and posterior roots of spinal nerves in rats has been described (11). The number of cutaneous nerves and neuropeptide content (substance P, calcitonin related peptide, vasoactive intestinal peptide, neuropeptide Y) is slightly decreased in indeterminate leprosy, reduced by 50% in lepromatous patients, and all but absent in tuberculoid leprosy lesions (10). Our results indicate that denervation seems not to be a significant factor in reducing epidermal cell proliferation, since this decrease is much greater in lepromatous patients than in tuberculoid patients, while denervation is much more extensive in tuberculoid than in lepromatous leprosy (10).
The reduction in epidermal cell proliferation in skin lesions of leprosy patients seems instead to be related to a deficiency in the cellular immune response. Differences in the cutaneous cellular immune responses in leprosy patients have been described (22). The cellular immune response deficiency is more intense in lepromatous leprosy where the greatest reduction in epidermal cell proliferation occurs. In indeterminate and tuberculoid leprosy, where the cellular immune response is less deteriorated, cell proliferation is also less reduced. When cell-mediated immunity in lepromatous skin lesions is reconstituted by intradermal injection of PPD, IFN- γ , and rlL2, keratinocytes increase in size and number, leading to epidermal thickening, while the cutaneous inflammatory process changes to a cell infiltrate characteristic of tuberculoid lesions (7-9). In reactional states (erythema nodosum leprosum) cutaneous lesions show epidermal hyperplasia (21). Keratinocytes secrete a variety of cytokines displaying immunological, inflammatory and proliferative activities (2,23). The deficiency in the cutaneous cellular immune response inducing a reduction in kcratinocytc numbers may thus lead to a decrease in cytokine secretion by keratinocytes themselves. This decrease in cytokine levels could possibly intensify the deficiency in the cellular immune response.
In 4 lepromatous patients both skin biopsies showed histopathologic lesions (Table 3); 2 of these patients showed rather similar percentages of epidermal cell proliferation in both biopsies, but the other 2 patients showed large differences between biopsies. These differences in cell proliferation suggest that areas with characteristic histopathologic features in the same patient may have varying impairment of the cellular immune response.
Percentages of labeled epidermal cells in areas without lesions in leprosy patients and in nonleprosy "normal" controls were similar. The decrease in epidermal proliferation in leprosy patients thus seems to be restricted to areas with cutaneous lesions.
The median percentage of the total number of labeled cells (basal + suprabasal) in skin biopsies from unaffected areas of leprosy patients and from "normal" nonleprosy patients found by us was 4.48%. Comparable numbers have been reported: 4.9% (3), 3.8% (1) and 2.5% (5,12,25). In our biopsies, labeling in the suprabasal layer was always greater (55%) than in the basal layer (45%). In skin biopsies from "normal" nonleprosy patients labeling also was greater in the suprabasal layer (51%) than in the basal layer (49%). Higher epidermal cell labeling in the basal layer (68%) than in suprabasal layer (32%) in normal human skin has been described (17). In areas with lesions from indeterminate patients, cell labeling was greater in the suprabasal layer than in the basal layer in 4 out of 5 biopsies. In tuberculoid patients, cell labeling in the basal layer was greater than in the suprabasal layer in 2 out of 4 patients. In lepromatous patients, cell labeling was greater in the basal layer than in suprabasal layer in 8 out of 12 biopsies.
Acknowledgment. The authors thank Mrs. Laura M. Kawasse and Marcia A. 0. Dcstido for their technical contributions. This work was supported in part by CNPq and FAPESP.
REFERENCES
1. ALLEGRA, F. and D E PANFILLIS, G. An in vivo method of studying the kinetics of cell proliferation in normal human epidermis. Acta Dermatol. 54 (1974) 87-90.
2. ANSEL, J., PERRY, P., BROWN, J., DAMN , D., PHAN, T., HART, C, LUGER, T. and HEFENEIDERS, S. Cytokine modulation of kcratinocytc cytokines. J. Invest. Dermatol. 94 (1990) 101S.
3. EPSTEIN, W . L. and MAIBACH, H. I. Renewal in human epidermis. Arch. Dermatol. 101 (1965) 323-328.
4. GODAL, T. Leprosy Immunology. Some aspects of the role of the immune system in the pathogenesis of disease. Lepr. Rev. 55 (1984) 407-414.
5. HELL, E. and MAIBACH, H. A. A comparison of in vivo and in vitro methods of identifying human epidermal cell in DNA synthesis. Br. J. Dermatol. 86 (1972) 506-507.
6. KAPLAN, G., KIESSLING, R., TEKLEMARIAN, S., HANCOCK, G., SHEFTEL, G., JOB, C. K., CONVERSE, P., OTTENHOFF, T. H. M., BECX-BLEUMINK, M., DIETZ, M. and COHN, Z. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interlcukin 2. J. Exp. Med. 169 (1989) 893-907.
7. KAPLAN, G., MATHUR, N. K., JOB, C. K., NATH, I. and COHN, Z. A. Effect of multiple interferon gamma injections on the disposal of Mycobacterium leprae. Proc. Natl. Acad. Sci. U.S.A. 86 (1989) 8073-8077.
8. KAPLAN, G., NUSRAT, A., SARNO, E. N., JOB, C. K., MCELRATH, J., PORTO, J. A., NATHAN, C. F. and COHN, Z. Cellular responses to the intradermal injection of recombinant human gamma interferon in lepromatous leprosy patients. Am. J. Pathol. 128 (1987) 345-353.
9. KAPLAN. G., WITMER. M. D., NATH, I.. STEINMAN, R.M., LAAL, S., PRASAD, H. K., SARNO, E. N., ELVERS, V. and COHN, Z. Influence of delayed immune reactions on human epidermal keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 83 (1986) 3469-3473.
10. KARANTH, S. S., SPRINGALL, D. R., LUCAS, S., LEVY, D., ASHBY, P., LEVENE, M. M. and POLAK, J. M. Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J. Pathol. 157 (1989) 1526-1537.
11. KULAGIN, A. N. Effect of division of anterior and posterior roots of spinal cord on mitotic activity of epidermis. Bull. Exp. Biol. Med. 49 (1960) 525-527.
12. LACHAPELLE, J. M. and GILLMAN, T. Tritiated thymidine labelling of normal human epidermal cell nuclei. Br. J. Dermatol. 81 (1969) 603-616.
13. OKHANDIAR, R. P., SINHA, E., SINHA, R. R. and MISHRA, A. D. Morphometric study of stratum corncum in leprosy. Ind. J. Lepr. 61 (1989) 49-53.
14. PALERMO, M. H. R., ZUCOLOTO, S., VUGMAN, I., FLEURY, R. N. and TRAD, E. S. Autoradiographic evidence of reduced epidermal cell proliferation in leprosy patients. Int. J. Lepr. 60 (1992) 283-285.
15. PEARSON, J. M. H. and Ross, W. F. Nerve involvement in leprosy. Pathology, differential diagnosis and management. Lepr. Rev. 46 (1975) 199-212.
16. PELC, S. R. The stripping film technique of autoradiography. Int. J. Appl. Radiol. 1 (1956) 172-178.
17. PENNEYS, N. S., FULTON, J. E., WEINSTEIN, G. D. and FROST. P. Location of proliferating cells in human epidermis. Arch. Dermatol. 101 (1970) 323-328.
18. REA, T. H., SHEN, J. Y. and MODLIN, R. L. Epidermal kcratinocytc la expression, Langcrhans' cell hyperlpasia and lymphocytic infiltration in skin lesions of leprosy. Clin. Exp. Immunol. 65 (1986) 253-259.
19. RIDLEY, D. S. and JOPLING, W . H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34 (1966) 255-273.
20. RISTOW, H. J. A major factor contributing to epidermal proliferation in inflammatory skin diseases appears to be intcrleukin 1 or a related protein. Proc. Natl. Acad. Sci. U.S.A. 84 (1987) 1940-1944.
21. SANTOS, I. 0., SUFFYS, P. N., BONIFACIO, K., MARQUES, M. A. and SARNO, E. N. In vitro tumor necrosis factor production by mononuclear cells from lepromatous leprosy patients and from patients with erythema nodosum leprosum. Immunol. Immunopathol. 3 (1993) 199-203.
22. SCOLLARD, D. M. Inside the skin; the local immune and inflammatory milieu in leprosy. Am. J. Trop. Med. Hyg. 44 (1991) 17S-23S.
23. TIGELAAR, R. E.. LEWIS, J. M . and BERGSTRESSER, P. R. TCR gamma/delta + dendritic epidermal T cells as constituents of skin associated lymphoid tissue. J. Invest. Dermatol. 94 (1990) 58S-63S.
24. WEDDELL, G., COWAN, M. A., PALMER, E. andRAMASWAMY, S. Psoriatic Skin. Arch. Dermatol.91 (1965) 252-266.
25. WEINSTEIN, G. D., MCCULLOUGH, J. L. and Ross,P. Cell kinetic basis for pathophysiology of pso-riasis. J. Invest. Dermatol. 45 (1965) 257-262.
26. WEINSTEIN, G. D. and VAN SCOTT, E. J. Auto-radiographic analysis of turnover times of normaland psoriatic epidermis. J. Invest. Dermatol. 45 (1965) 257-262.
1. M.D., Ph.D., Department of Pathology; Faculty of Medicine of Ribeirão Preto, University of Sao Paulo, 14049- 900 Ribeirão Preto, S.P., Brazil.
2. M.D., Ph.D., Department of Pathology; Faculty of Medicine of Ribeirão Preto, University of Sao Paulo, 14049- 900 Ribeirão Preto, S.P., Brazil.
3. M.D., Ph.D., Department of Biochemistry, Faculty of Medicine of Ribeirão Preto, University of Sao Paulo, 14049- 900 Ribeirão Preto, S.P., Brazil.
4. M.D., Ph.D., Department of Pathology, Faculty of Odontology, Bauru, Brazil.
Reprint requests to Dr. Zucoloto at the above address or FAX 55-16-633-1068.
Received for publication on 2 May 1995;
Accepted for publication in revised form on 29 November 1995.