- Volume 64 , Number 1
- Page: 44–50
Viability of Mycobacterium leprae in skin and peripheral nerves and persistence of nerve destruction in multibacillary patients after 2 years of multidrug therapy
ABSTRACT
The pathological changes, bacterial load, and viability of Mycobacterium leprae in the skin and nerves of nine lepromatous leprosy patients who had undergone 2 years of multidrug therapy (MDT) were studied. M. leprae and varying amounts of their remnants were present in the nerves and skin of all but one patient. M. leprae isolated f rom skin biopsies of six patients and nerve biopsies of nine patients were inoculated into mouse foot pads. No growth was obtained f rom any one of them. During the electron-microscopic examination of three nerve biopsies, only one specimen showed a small number of solid-staining M. leprae. These findings would explain the low relapse rate in patients treated with 2 years of lix-duration MDT. Results of a long-term follow up of patients is awaited with interest. The possibility of nerve paralysis due to intraneural microreaction and fibrosis consequent to the continued presence of dead bacterial remnants should be seriously considered.RÉSUMÉ
On a étudié les modifications pathologiques, la acharge bactérienne, et la viabilité de Mycobacterium leprae dans la peau et les nerfs de neuf patients lépromateux qui avaient suivi deux ans de polychimiothérapic (PCT). M. leprae et des quantités variables de leurs résidus étaient présents dans les nerfs et la peau de tous les patients, à l'exception d'un seul. Des M. leprae isolés de biopsies cutanées provenant de six patients et de biopsies de nerfs de neuf patients ont été inoculés dans le coussinet plantaire de souris. On n'a obtenu aucune croissance pour aucune d'entre elles. Lors de l'examen par microscopic électronique de trois biopsies nerveuses, seul un spécimen a montré un petit nombre de M. leprae uniformément colorés. Ces observations expliqueraient le faible taux de récidive chez les patients traités par une PCT de durée fixe de deux ans. Les résultats d'un suivi à long terme des patients sont attendus avec intérêt. La possibilité d'une paralysie nerveuse duc à une microréaction intraneurale et à la fibrose consécutive à la présence continue de résidus de bactéries mortes devrait être sérieusement prise en considération.RESUMEN
Se estudiaron los cambios patológicos, la carga bacteriana y la viabilidad de Mycobacterium leprae en la piel y los nervios de 9 pacientes lepromatosos que habían recibido poliquimioterapia (POT) durante 2 años. M. leprae y cantidades variables de sus residuos estuvieron presentes en la piel y nervios de todos los pacientes menos en uno. Las bacterias aisladas de las biopsias de piel de 6 pacientes y de las biopsias de nervio de 9 pacientes fueron inoculadas en las almohadillas plantares del ratón. En ningún caso se obtuvo crecimiento. En el examen por microscopía electrónica de 3 biopsias de nervios, sólo un espécimen mostró un pequeño número de M. leprae con tinción sólida. Estos hallazgos explicarían la baja frecuencia de recurrencias en los pacientes tratados por 2 años con PQT. Se esperan con interés los resultados de un seguimiento más prolongado de los pacientes. Por otro lado, debe considerarse muy seriamente la posibilidad de parálisis neural debida a la microrreacción intraneural y a la fibrosis resultante de la continua presencia de los remanentes bacilares.Multidrug therapy (MDT) instituted by the World Health Organization (WHO) in 1982 (13) has made great contributions to the management and control of leprosy. It has very much shortened the duration of therapy and, thereby, it has enhanced the compliance of patients in taking treatment. It has reduced to a minimum the development of drug-resistant organisms. The efficacy of therapy to cure any disease is assessed by the relapse rate. The reported relapse rate following MDT until recently has been very low (12). In a trial of fixed-duration MDT administered for 2 years to previously untreated multibacillary (MB) patients, no relapses were noticed until 1994 (personal communication, K. Jesudasan).
Relapses ordinarily occur consequent to Mycobacterium leprae which lie dormant and have survived in the tissues even at the end of 2 years of MDT, or are due to bacilli which are resistant to the drugs administered or to ineffective treatment as a result of irregular drug intake. In this study, the viability of M. leprae and the pathological changes of the skin and nerves of MB patients who had undergone 2 years of MDT were assessed.
MATERIALS AND METHODS
In the St. Thomas Hospital and Leprosy Center, Chettupattu, India, there are many MB patients belonging to both polar lepromatous (LL) and borderline lepromatous (BL) groups who had undergone 2 years of fixed-duration MDT. Patients chosen for this study received a drug regimen consisting of supervised once-monthly doses of rifampin (600 mg) and clofazimine (300 mg) and unsupervised, self-administered, daily intake ofdapsonc (100 mg) and clofazimine (50 mg) for a total period of 24 months. Adequate treatment implied 24 monthly doses of the combined therapy within 36 months. There were eight patients with a bacterial index (BI) > 2+ who had regularly taken 24 monthly doses of combined therapy in as many months, and one patient who had taken the 24 monthly doses in 28 months. All nine patients were investigated within 4 weeks of completing therapy. After a thorough clinical examination, skin smears were done to assess the BI. A skin lesion which appeared active and the thicker of two radial cutaneous nerves were biopsied. Both the skin and nerve biopsies from all nine patients except three were divided into two portions; one was used for histopathologic study, the other was processed for mouse foot pad inoculation. In three patients the nerve biopsies were divided into three portions; one was used for histopathologic examination, another was processed for electron-microscopic study, the third was used for mouse foot pad work.
Skin smears
Slit-skin smears were prepared from six routine sites, and if the lesions were patchy in distribution, one more smear was taken from an active skin lesion. The BI was calculated according to Ridley's scale (6).
Histopathologic study
Both skin and nerve biopsies were fixed in 10% neutral buffered formalin for 24 to 48 hr and processed for paraffin blocks. Five sections were made; one section was stained with hematoxylin and eosin (H&E) and another with a modified Fite stain for M. leprae (2). The nature of the granuloma present and the bacterial load of all the skin and nerve biopsies were assessed. The granuloma fraction (GF) in the skin lesions was estimated.
Electron-microscopic study
The nerve specimens obtained from three patients were cut into several 1-mm cubes, fixed in 3% glutaraldehyde at 4ºC for 4 hr, postfixed in osmium tetroxide, processed, and embedded in araldite. Ultrathin sections were made, stained with lead citrate and uranyl acetate, and were examined with a Philips EM 201C electron microscope.
Mouse foot pad study
All of the nine skin and nine nerve biopsies were processed for studying the viability of M. leprae by growth in the foot pads of normal Swiss albino mice (8). In three skin biopsies no organisms were isolated during processing, and they were discarded. From all other specimens a saline suspension of M. leprae containing 104 organisms in 0.03 ml were inoculated into both foot pads of a group of five Swiss albino mice. The foot pads were harvested at the end of 6 months and 9 months in all experiments, except one in which only the result at the end of 6 months was available.
RESULTS
Clinical picture
Of the nine patients studied, 6 were males and 3 females, and their ages ranged from 28 to 55 years (mean 36.5 years). Six patients belonged to the LL group and three to the BL group (The Table). All six LL patients showed evidence of diffuse infiltration of the skin, with some areas in the trunk having fine scales. Both ulnar and common peroneal nerves were thickened to varying degrees in all of the six LL patients. One patient had ulnar and median nerve paralysis of the left hand, ulnar palsy of the right hand, and clawing of the toes of the left leg. The other patients had no clinical evidence of motor paralysis. All of them had "gloveand-stocking" anesthesia of both upper and lower extremities. The three BL patients had multiple, generalized, symmetrical patchy lesions of various sizes over their trunks and extremities. None of them had clinical evidence of motor paralysis. However, extensive impairment of sensation was present in all of the lesions and in both the lower and upper extremities.
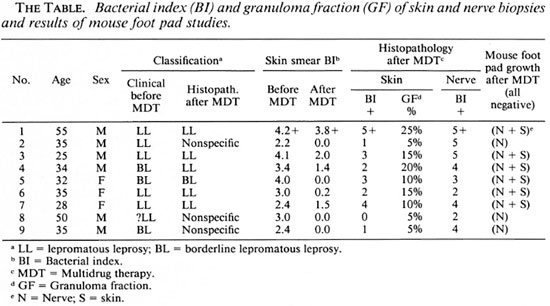
During the 2-year period of MDT, all patients showed signs of resolution, such as wrinkling of skin and scaling over the lesions. None of them developed reactions. However, one LL patient, who although never complaining of pain or tenderness of nerves, gradually and imperceptibly developed "silent left ulnar palsy" while taking MDT.
Skin smears
Skin smear values before treatment ranged from 2.2+ to 4.2+ with a mean of 3.2 + . At the end of MDT, three patients were BI negative; in the others it varied from 3.8 + to 0.2+ (mean 0.98 + ) (The Table).
Histopathology
Skin. The biopsies were performed only at the end of MDT and prctreatment biopsies were not available. In the skin lesions of two LL and one BL patients the GF was < 5% and only nonspecific inflammation composed of mononuclear cells, which included lymphocytes and histiocytes, was seen. In the other six patients the GF was between 10% and 25%, and foamy macrophages persisted in the granuloma in varying amounts. The BIS varied from 0 to 5 + (mean value 2.3 + ). In one patient the biopsy was negative for acid-fast bacilli (AFB). In five patients AF B were present only inside parenchymal cells, such as Schwann cells, endothelial cells and cells of the hair follicles. In only three patients did AF B persist inside macrophages in the granuloma and of these, two had an initial skin-smear BI of > 4 + . All of the bacilli present were broken and granular.
Nerves.
All of the nerve biopsies showed diffuse infiltration of the entire tissue with varying numbers of lymphocytes and foam cells. There was fibrous replacement of normal nerve parenchyma. It was difficult to differentiate the foamy macrophages from foamy Schwann cells. The perineurium was thickened and showed well marked fibrosis. Acid-fast staining showed clumps of bacilli inside many foam cells in all biopsies. A few scattered organisms were present in the perineurial cells, endothelial cells and Schwann cells. All of the bacilli were broken and granular. The Bis varied from 2+ to 5+ (mean 4.3 + ).
Electron microscopy
An electron-microscopic study of the radial cutaneous nerves in two LL and one BL patients was most informative and interesting. The normal appearance of the intrafascicular architecture was destroyed, and there was considerable loss of myelinated fibers and marked increases in endoneurial collagen fibrils (Figs. 1, 2 and 5). A few macrophages and many Schwann cells showed numerous intracellular M leprae. They were found inside large phagolysosomes containing much electron-transparent material (Figs. 1 and 2). In 2 of the 3 patients studied all bacilli present were fragmented and granular (Fig. 1), and in the third patient, after a prolonged search through many fields, a few solid-staining organisms were detected (Fig. 2). Many axons were seen in the process of undergoing active demyclination (Fig. 3). There were also several Bungner's bands (Fig. 4), indicating Schwann cell hyperplasia. Several poorly formed onion bulbs (Fig. 5), the result of repeated demyelination and remyelination of axons, were also present. An intraneural venule showed marked edema of the endothelial cells which almost blocked the lumen (Fig. 6). The perineurium showed many layers of thinned out and atrophied perineurial calls with a marked increase in intercellular collagen fibrils (Fig. 7). All findings indicated that although most of the bacilli were nonviable, the inflammation and the consequent destructive changes in the nerve were very active.
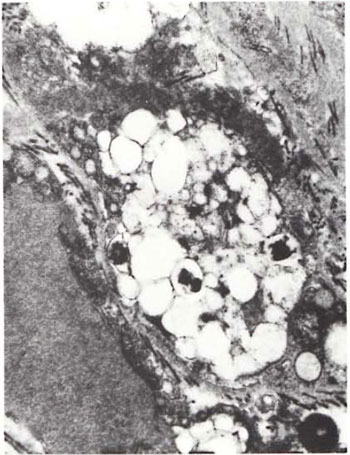
Fig.1. Portion of foamy Schwann cell with largephagolysosome containing several broken and granularM. leprae. Numerous collagen fibrils surround the cell ( x 24,000).
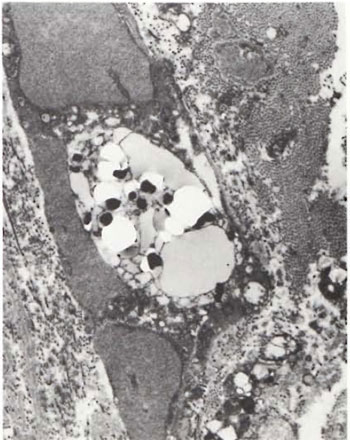
Fig. 2. Portion of foamy Schwann cell with largephagolysosome containing a few solid-staining M. leprae lying in a bed of electron-transparent material. Note well-marked increase in endoneurial collagen fibrils (x 10,000).
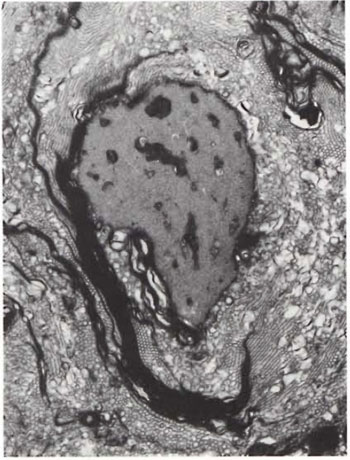
Fig. 3. A myclinated axon undergoing active de-myelination ( x 13,000).
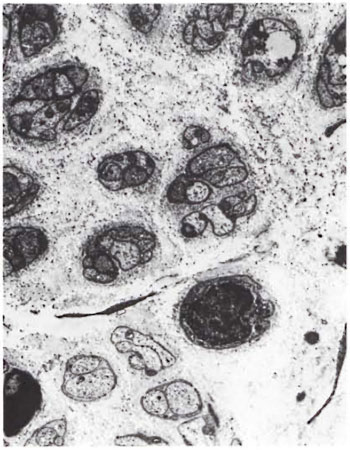
Fig. 4. Disorganized endoneurium with many Bungner's bands (x4000).
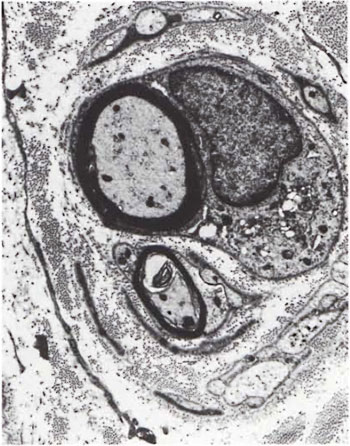
Fig. 5. Two myelinated axons surrounded by several flattened Schwann cell processes resembling an "onion bulb." Note numerous collagen fibrils in between the cell processes ( x6500).
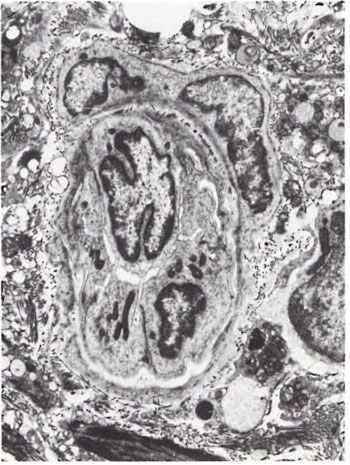
Fig. 6. Intraneural venule with marked edema ofendothelial cells causing occlusion of the lumen( x 3000).

Fig. 7. Perineurium with many layers of thinned out perineurial cells and a marked increase in inter-cellular collagen fibrils ( x 6500).
Mouse foot pad study
From all nine nerve biopsies and from the six skin biopsies in which countable M. leprae were obtained, bacilli were inoculated into the foot pads of Swiss albino mice. The results of the harvest were known at the end of 6 months and 9 months in all experiments, and at the end of 6 months in one experiment. No growth was obtained in any of them.
DISCUSSION
Clinically, all nine patients showed signs of resolution, although there was not complete inactivity of all lesions. An asymptomatic, insidious paralysis of an ulnar nerve in one patient was recorded almost at the end of the treatment period. In mass treatment programs, in which regular and periodical individual attention by well-trained leprosy workers was not always feasible, an asymptomatic paralysis of gradual onset could easily go unnoticed. Therefore, it is imperative to impress upon the staff to regularly and periodically assess motor functions of all nerves that are ordinarily affected so that an early impairment of nerve function can be detected and prompt treatment can be instituted. It is even better to educate the patient to look for a progression of anesthesia and for the onset of early paralysis so that he could bring it to the attention of a paramedical worker at the earliest possible opportunity.
Skin smears in these nine patients registered a steady decline at the rate of approximately 1 log per year except in two patients. Of these two, one showed a marked reduction from 4.0+ to 0+; the other had only a small reduction from 4.2+ to 3.8 + . The first patient was of the BL category, and such a sharp fall in BI in BL patients is not unusual. The persistence of a high BI in the other patient cannot be easily explained, especially when M. leprae isolated from this patient did not grow in the foot pads of normal mice. All nine patients arc being carefully followed.
In the histopathological study, persisting lepromatous granuloma was present in varying degrees in the skin lesions of six patients and in the nerve lesions of all nine patients. It is well known that the peripheral nerves serve as an immunologically protected site for M. leprae (1,4,6). Therefore, when M. leprae gain entry into the nerve, they seem to multiply more freely and more abundantly than in the other tissues. As a result, nerve lesions tend to downgrade as compared to skin lesions (11).
At the close of MDT a large number of bacilli were left behind in both the skin and the nerve lesions. As demonstrated in this study, a much larger load of M. leprae (mean value 4.3+) remained in the nerves as compared to the skin lesions (mean value 2.3 + ). However, M. leprae isolated from both skin and nerves from all nine patients did not grow in the foot pads of mice. It is quite possible that the peripheral nerve with a tight perineurium and a well-known bloodnerve barrier might have retarded the clearance of dead M. leprae. In a study reported earlier, of the 10 MB patients studied, viable bacilli were isolated from the skin of two and the nerves of three patients (9). In that study, immunologically compromised mice were used, and the inoculum into the foot pads consisted of 105 M. leprae. There might have been persisting viable M. leprae in the skin and nerves of these MB patients at the close of 2 years of MDT, but they were too small in number to be picked up in a study using normal mice in which the inoculum consisted of a maximum of only 104 bacilli. This might explain why relapses in MB patients reported to date have been almost negligible (12). Reports on longer-term follow up are awaited with great interest.
The electron-microscopic study showed evidence of a marked increase in fibrous tissue (Figs. 1, 2, 4 and 5), active demyelination (Fig. 3), signs of repeated demyelination and remyelination (Fig. 5), regeneration of Schwann cells (Fig. 4), and persistence of fragmented and degenerating M. leprae (Fig. 1) in all patients, and a few, rare, solid-staining organisms in one patient (Fig. 2). In addition there was marked thickening of the perineurium and proliferation of perineurial cells and a large increase in perineurial fibrous tissue (Fig. 7).
It is possible that a small number of M. leprae, too few to be detected by a mouse foot pad study using normal mice, may linger in nerves of a few patients. Their role in causing a relapse at a much later date and in bringing about gradual destruction of nerves, causing impairment of function, is real and needs further investigation. Also, dead and fragmented bacterial remnants were present in all nerve biopsies in fairly large quantities. Hopefully, in a majority of patients these bacillary fragments would gradually disappear without causing any damage to the nerves. However, in some patients they might be responsible for continuing the inflammatory process, causing destruction of nerve tissue long after therapy was discontinued (3,10). It is also possible that over time focal microreactions in nerves in response to bacillary antigens could cause nerve destruction (7).
In the present study there was significant fibrosis of the perineurium and endoneurium. Scarred nerves may cause anoxia to surviving nerve parenchyma, resulting in necrosis of axons (3). Therefore, it is imperative to carefully and periodically monitor patients released after MDT, especially those with obvious nerve involvement, for signs of paralysis and to institute treatment before irreversible paralysis is produced. In the management of patients who are potentially liable to develop nerve paralysis, a course of steroid therapy during the first 6 months of the 2-year course of MDT should be seriously considered.
Acknowledgment. Financial support for this study received from the American Leprosy Mission International and is gratefully acknowledged. We are grateful to Mr. K. Rajanna, Mr. P. Pugazendhi and Mrs. Rita Edward for the excellent technical assistance and to Miss K. Jayanthi for secretarial help.
REFERENCES
1. BODDINGIUS, J. Mechanisms of peripheral nerve damage in leprosy; electron and light microscope studies in patients throughout the spectrum. Quad. Coop. Sanit. (Bologne) - (1982) 65-84. As quoted by Ridley, D. S. In: Pathogenesis of Leprosy and Related Diseases. London: Butterworth and Co., 1988, p. 78.
2. JOB, C. K. andCHACKO, C. J. G. A modified Fite's stain for demonstration of M. leprae in tissue sections. Indian J. Lepr. 68(1986)17-18.
3. JOB, C. K.., VICTOR, D. B. I. and CHACKO, C. J. G. Progressive nerve lesion in a disease arrested leprosy patient -an electron microscopic study. Int. J. Lepr. 45(1977)255-260.
4. PEARSON, J. M. H. and Ross, W. F. Nerve involvement in leprosy; pathology, differential diagnosis and principles of management. Lepr. Rev.46(1975)199-212.
5. RIDLEY, D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Bristol: John Wright and Sons Ltd., 1964, pp. 620-622.
6. RIDLEY, D. S. and RIDLEY, M. J. Classification of nerves is modified by delayed recognition of M. leprae. Int. J. Lepr. 54(1986)596-606.
7. RIDLEY, M. J., WATERS, M. F. R. and RIDLEY, D. S. The events surrounding the recognition of M. leprae in nerves. Int. J. Lepr. 55(1987)99-108.
8. SHEPARD, C. The experimental disease that follows the infection of human leprosy bacillus into foot pads of mice. J. Exp. Med. 112(1966)445-454.
9. SHETTY, V. P., SUCHITRA, K., UPLEKAR, M. W. and ANTIA, N. H. Persistence of M. leprae in the peripheral nerves as compared to the skin of multidrug treated leprosy patients. Lepr. Re v. 63(1992)329-336.
10. SHETTY, V. P., UPLEKAR, M. W. and ANTIA, N. H. Immunohistological localization of mycobacterial antigens within the peripheral nerves of treated leprosy patients and their significance to nerve damage in leprosy. Acta Ncuropathol. 88(1994)300-306.
11. SRINIVASAN, H., RAO, K. S. and IYER, C. G. S. Discrepancy in the histopalhological features of leprosy lesions in the skin and peripheral nerve; report of a preliminary study. Indian J. Lepr. 54(1982)275-282.
12. WATERS, M. F. R. Relapse following various types of multidrug therapy in multibacillary leprosy. (Editorial) Lepr. Rev. 66(1995)1-9.
13. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.D., F.R.C.Path., Consultant Pathologist; St. Thomas Hospital and Leprosy Center, Chettupattu 606801, T.S. District, Tamil Nadu, India.
2. M.B.B.S., D.T.M.&H.. Head, Department of Leprosy; St. Thomas Hospital and Leprosy Center, Chettupattu 606801, T.S. District, Tamil Nadu, India.
3. M.D., Chief Medical Oflicer, St. Thomas Hospital and Leprosy Center, Chettupattu 606801, T.S. District, Tamil Nadu, India.
4. M.D., Ph.D., Professor of Pathology, Christian Medical College & Hospital. Vellore 632004, Tamil Nadu, India.
Received for publication on 28 August 1995.
Accepted for publication in revised form on 30 November 1995.