- Volume 64 , Number 1
- Page: 82–4
Radiometric procedure for detecting a cultivable Mycobacterium in Mycobacterium leprae-lnfected armadillo tissue
To the Editor:
For the past two decades, experimentallyinfected armadillo tissues have been the principal source of Mycobacterium leprae for microbiological investigations. The results of earlier studies have revealed the presence of armadillo-derived mycobacteria (ADM) in some armadillos infected with M. leprae (4,5). Contamination of purified M. leprae suspensions with ADM would undoubtedly interfere with any research exploiting the unique characteristics of M. leprae. In this communication, we describe a rapid radiometric procedure for detecting a mycobacterial species other than M. leprae in armadillo tissues.
Earlier in vitro studies have demonstrated the ability of M. leprae to significantly oxidize 14C-palmitic acid when incubated in an axenic medium (2). However, previous data from our laboratory (unpublished observations) provided evidence that M. leprae, unlike most other organisms, does not utilize exogenous14C-acetate in vitro to any measurable degree for fatty acid synthesis or oxidation. Based on this finding, it was possible to add 14C-acetate to armadillo tissue homogenates containing M. leprae or cultivable mycobacteria and compare acetate utilization in one particular reaction (e.g., oxidation to carbon dioxide).
Noncontaminated liver tissue was obtained from a naive armadillo and a 10% homogenate was prepared in Middlebrook 7H9 complete culture medium. M. leprae suspensions were prepared from experimentally infected, athymic nude mice as previously described (3). A strain of M. avium, previously isolated from armadillo tissue experimentally infected with M. leprae, was cultivated in medium as described above. Aliquots (1.0 ml) of liver homogenate were distributed into small screwcapped vials and inoculated with appropriate bacterial suspensions as outlined in The Figure. Control vials contained only culture medium in place of liver homogenate. Each vial was pulsed with 0.5 µ Ci of 14C-acetate (58.2 mCi/mmole), capped loosely, and transferred to wide-mouthed scintillation vials. The viability of M. leprae was assessed in the control medium by incubating aliquots of the organism in the presence of 0.5 µCi 14C-palmitic acid (850 mCi/mmole).
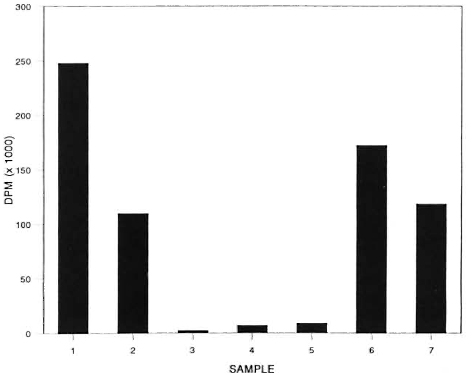
The figure. Evolution of 14CO2 from labelled substrate incubated with M. leprae and M. avium for 5 days under the following experimental conditions: 1 = 107 M. avium , in medium, + 14C-acetate; 2 = 108 M. leprae , in medium, + 14C-paImitate; 3 = 108 M. leprae + 14C-acetate; 4 = 108 M. leprae + liver homogenate + 14C-acetate; 5 = liver homogenate + 14C-acetate; 6 = 107 M. avium + 108 M. leprae + liver homogenate + 14C-acetate; 7 = 107 M. avium + liver homogenate + 14C-acetate.
The evolution of 14CO2was measured at suitable time intervals using a method described by Buddemeyer, et al. (1). Briefly, 14CO2 released was trapped on filterpaper strips previously soaked with a specially prepared liquid scintillation solution, air dried, and placed inside the counting vials. The vessels containing only M. leprae were incubated at 33ºC; those containing M. avium were incubated at 37ºC.
The data depicted in The Figure compare the release of 14CO2 from radioactive substrate incubated in the presence of various combinations of armadillo homogenate, M. leprae and M. avium overa period of 5 days.
An increase in the release of 14CO2 was observed in liver homogenate and medium incubated with M. avium.
A similar result was noted with homogenate incubated with a combination of M. avium and M. leprae. By contrast, the addition of M. leprae to the liver homogenate or M. leprae incubated in medium alone did not significantly alter 14CO2 production. It was interesting to note a reduction in the amount of 14CO2released in the suspensions of liver homogenate containing M. avium, hypothetically, from substrate competition or inhibition by tissue components. Since significant amounts of 14CO2were released when M. leprae were inoculated in the presence of 14CO-palmitate, we may conclude that the bacilli were metabolically active.
The outcome of this study is consistent with our previous observation on the apparent inability of M. leprae to utilize exogenous 14C-acetate. We have already emphasized the importance of obtaining armadillo-derived M. leprae free from contamination by other mycobacteria. By exploiting the failure of M. leprae to actively metabolize 14C-acetate under these experimental conditions, we present a possible method for rapidly distinguishing M. leprae from this potential mycobacterial contaminant.
- Eugene B. Harris, B.S.
Rita M. Sanchez, B.S.
Laboratory Research Branch
GWL Hansen's Disease Center at Louisiana State University
P.O. Box 25072
Baton Rouge, LA 70894, U.S.A.
REFERENCES
1. BUDDEMEYER, E., HUTCHINSON, R. and COOPER, M. Automatic quantitative radiometric assay of bacterial metabolism. Clin. Chem. 22(1976)1459-1464.
2. FRANZBLAU, S. Oxidation of palmitic acid by At. leprae in an axenic medium. J. Clin. Microbiol. 26(1988)18-21.
3. FRANZBLAU, S. and HASTINGS, R. Rapid in vitro metabolic screen for antileprosy compounds. Antimicrob. Agents Chemother. 31(1987)780-783.
4. LARSSON, L., DRAPER, P. and PORTAELS, F. Use of gas chromatography to differentiate Mycobacterium leprae from cultivable armadillo-derived mycobacteria, M. avium/intracellulare end M. lepraemurium by analysis of secondary alcohols. Int. J. Lepr. 53(1985)441-446.
5. PORTAELS, F. and PATTYN, S. R. Isolation of fastidiously growing mycobacteria from armadillos infected with Mycobacterium leprae. Int. J. Lepr.50(1982)370-374.