- Volume 64 , Number 1
- Page: 69–78
IgG subclass recognition pattern in leprosy: recognition of M. leprae antigens by lgG1 and lgG3 antibodies is distinct across the disease spectrum
ABSTRACT
The recognition of Mycobacterium leprae antigens by IgG subclasses in patients with leprosy was investigated by electrophoresing M. leprae sonicate in SDS-polyacrylamide gel and immunoblotting analysis. Serum pools were used f rom leprosy patients with cither lepromatous (LL/BL) or tuberculoid (BT/TT) disease. A serum pool f rom healthy controls (EC) was used to determine the baseline antibody activity. To adjust for quantitative differences in antibodies across the disease spectrum, the LL/BL serum pool was used at a 1:200 dilution; the BT/TT serum pool, at 1:20 dilution. Monoclonal antibodies specific for each of the IgG subclasses were used as probes, with anti-mouse IgG conjugated to alkaline phosphatase as the revealing probe. IgG1 antibodies bound to several discrete bands in the range of 10-70 kDa in LL/BL patients, while BT/TT patients showed a more diffuse pattern with the strongest IgG1 antibody binding in the region of 25-40 kDa. Recognition with IgG2 was restricted to a region between 25-36 kDa (which also stained strongly for carbohydrates) in both LL/BL and BT/TT patients. Binding with IgG3 antibodies was more restricted than IgG1 antibodies in LL/ BL sera with strong recognition restricted to 25 and 28 kDa. BT/TT sera showed strong binding with IgG3 antibodies in the region of 25-32 as well as 5-7 kDa. IgG4 antibodies showed weak binding to a 28-kDa in lepromatous patients only. The différences in IgG subclass récognition patterns and their implications are discussed.RÉSUMÉ
On a étudié, par électrophorèse de sonicats de Mycobaclerium leprae dans du gel de SDS-polyacrylamide et par immunoblotting, la reconnaissance des antigènes de M. leprae par des sous-classes d'IgG chez, des malades de la lèpre. On a utilisé des pools de serum provenant de malades de la lèpre, soit du type lépromatcux (LL/BL), soit du type tuberculoide (BT/TT). On a utilisé un pool de serum de témoins en bonne santé afin de déterminer l'activité de base des anticorps. Afin d'ajuster pour les différences quantitatives d'anticorps tout le long du spectre de la maladie, le pool de serum LL/BL a été utilisé à une dilution de 1:200 et le pool de serum BT/TT à une dilution de 1:20. Des anticorps monoclonaux spécifiques pour chacune des sous-classes d'IgG ont été utilisées comme sondes, avec des IgG anti-souris conjugués à de la phosphatase alkaline comme sonde de révélation. Des anticorps IgGl se lièrent à dillércntes bandes discrètes dans des régions allant de 10 à 70 kDa chez les patients LL/BL, tandis que les malades BT/TT montraient un type plus diffus avec la plus forte liaison anticorps IgG1 dans la région 25-40 kDa. La reconnaissance avec l'IgG2 a été limitée à une région entre 25 et 36 kDa (qui avait également une forte coloration pour les carbohydrates) chez les malades LL/BL et chez les BT/TT. La liaison avec les anticorps IgG3 était plus limitée que pour les anticorps IgG1 aux scrums LL/BL, avec une forte reconnaissance limitée aux 25 et 28 kDa. Les scrums BT/TT ont montré une forte liaison avec les anticorps IgG3 dans la région 25-32 kDa aussi bien que dans celle de 5-7 kDa. Les anticorps IgG4 ont montré une faible liaison dans la région de 28 kDa seulement chez les malades lépromatcux. Les différences dans les types de reconnaissance de sous-classes d'IgG, ainsi que leurs implications, sont discutées.RESUMEN
Se investigó el reconocimiento de antigenos de Mycobacterium leprae por anticuerpos IgG de pacientes con lepra utilizando las técnicas de electroforesis en gel de poliacrilamida y de inmunoelcctrotransferencia. Se usaron mezclas de sueros de pacientes con lepra lepromatosa (LL/BL) y de pacientes con lepra tuberculoide (BT/TT). También se usó una mezcla de sueros de individuos sanos (CS) para determinar la actividad basai de anticuerpo. Para corregir las diferencias cuantitativas en anticuerpos a lo largo del espectro de la enfermedad, la mezcla de sueros LL/BL se usó a la dilución 1:200 y la mezcla de sueros BT/TT, a la dilución 1:20. Como sondas se utilizaron anticuerpos monoclonales especificos para cada subclase de IgG y el revelado se hizo con anticuerpos anti-IgG de ratón conjugados con fosfatasa alcalina. Mientras que en los pacientes con LL/BL los anticuerpos IgG1 se enlazaron a varias bandas discretas en el rango de los 10-70 kDa, en los pacientes BT/TT el patrón de reactividad fue más difuso y el cnlazamicnto más fuerte de los anticuerpos IgG1 ocurrió en la región de los 25-40 kDa. Tanto en los pacientes LL/BL como en los pacientes BT/TT, el reconocimiento con anticuerpos IgG2 estuvo restringido a la región entre los 25 y los 40 kDa (la cual también se tiñe fuertemente para carbohidratos). En los sueros LL/BL, el cnlazamicnto de los anticuerpos IgG3 fue más restringido que el de los anticuerpos IgG1, con fuerte reactividad con componentes de 25 y 28 kDa. Los sueros BB/TT mostraron fuerte cnlazamicnto con anticuerpos IgG3 en las regiones de 25-32 y de 5-7 kDa. Los anticuerpos IgG4 mostraron enlazamiento débil con un componente de 28 kDa sólo en los pacientes lepromatosos. Se discuten las diferencias en los patrones de reconocimiento de las subclases de IgG y sus implicaciones.Mycobacterium leprae, the causative agent of leprosy, has a complex antigenic structure (5). In order to understand the disease pathogenesis, it is important to understand the role that these antigens play in evoking the immune response in leprosy. There is an inverse relationship between antibody and T-cell responses in leprosy. Lepromatous patients with the more disseminated form of disease have a higher bacterial load, high levels of antibodies, and low-to-absent T-cell responses, while tuberculoid leprosy patents, with a more localized form of the disease and a low bacterial load, demonstrate high cellular response and low concentrations of antibodies (8,16). Among the isotypes, IgG is the predominant circulating antibody against M. leprae (14). Human IgG is composed of four subclasses which differ in their structure and biological activities, such as complement fixation (2) and binding to effector cells which may have important pathogenetic implications. The switching of IgM antibody response to one of the four IgG subclass antibodies requires cytokines secreted by different helper T cells (15,21). Although M. leprae -specific IgG and IgM antibody responses have been studied extensively, both qualitatively and quantitatively, very little information is available on IgG subclass antibodies in leprosy (7,11,25).
To understand the relationship between differential T-cell activation and IgG subclass antibody responses in leprosy, we have been analyzing the IgG subclass antibody response across the leprosy spectrum. IgG subclass antibodies as assessed by ELISA were selectively raised for IgG1, IgG2 and IgG3 antibodies to M. leprae sonicate (11); whereas only IgG1 and IgG3 antibodies were detected to a purified recombinant protein antigen, M. leprae 18 kDa (10). Furthermore, the distribution of IgG subclass antibodies across the disease spectrum differed only in quantitative terms, with higher concentrations toward the lepromatous pole(10,11). In this report we have, for the first time, described the recognition pattern of M. leprae antigens by the four subclasses of IgG antibodies, across the leprosy disease spectrum, using immunoblotting analysis. The major difference in the recognition of M. leprae antigens in the two polar groups was observed with IgG1 and IgG3 antibodies which may be related to differential T-cell activation across the disease spectrum.
MATERIALS AND METHODS
Study subjects
Patient material was collected from newly diagnosed, untreated leprosy patients at the Marie Adelaide Leprosy Center in Karachi, Pakistan. The patients were classified as lepromatous (LL), borderline lepromatous (BL), borderline tuberculoid (BT) and tuberculoid (TT) leprosy by the standard clinical signs. The clinical classification of each patient was confirmed by histopathology according to the standard criteria as described by Ridley (18). Patients with reactional complications were excluded from the study. The control group consisted of 10 healthy individuals living in the endemic area of Karachi, with no known exposure to leprosy, coming from varied socioeconomic background.
Serum
Five ml of peripheral blood was obtained from each of the patients and control subjects. The blood was allowed to clot overnight at 4ºC, The serum was removed and centrifuged at 400 x g for 15 min; the clear supernatc was distributed in 100- µ d aliquots and kept at -70ºC until use.
Reagents
Monoclonal antibodies specific for human IgG subclasses were HP6069 (anti-IgG1) kindly provided by Dr. Hamilton, Johns Hopkins University, Baltimore, Maryland, U.S.A.; HP6002 (anti-lgG2), HP6047 (anti-IgG3), HP6023 (anti-IgG4), prepared at the Centers for the Disease Control and Prevention, Atlanta, Georgia, U.S.A., and were a gift from Dr. Reimer. Goat anti-mouse IgG conjugated to alkaline phosphatase was purchased from Jackson Immuno Research Laboratories, West Grove, Pennsylvania, U.S.A., and was used according to the manufacturer's recommendations. Bovine serum albumin (BSA), 5-bromo 4-chloro 3-indolyl phosphate (BCIP), para-nitro-phenyl phosphate, β -mercaptoethanol, Tris hydroxy methyl amino methane and Schiff's reagent' were obtained from Sigma Chemical Co., St. Louis, Missouri, U.S.A. Acrylamidc, bisacrylamide, sodium dodecyl sulfate (SDS), glycine, ammonium persulfatc, Coomassie blue and nitrocellulose membrane (0.45 µ m) were purchased from Bio-Rad Laboratories, Richmond, Virginia, U.S.A.
Quantitative determination of M. lepra e specific IgG subclasses
ELISAs were carried out on 96-well, flatbottom, polystyrene Immulon 4 plates (Dynatech, Chantilly, Virginia, U.S.A.) as described in detail previously (11). Briefly, M. Leprae -soluble sonicate (batch CD114, kindly provided by Dr. R. J. W. Rees, National Institute of Medical Research, London) was coated at 0.4 µ .g/well in 100 µ l of carbonate buffer at pH 9.6, incubated for 2 hr at 37ºC and additionally incubated at 4ºC overnight. The plates were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) and the remaining binding sites were blocked with 5% BSA in PBS at 37ºC for 2 hr. Reference and test sera diluted in PBS-T containing 1% BSA were added to the wells at doubling dilutions. The sera were incubated for 2 hr at 37ºC followed by an overnight incubation at 4ºC. Monoclonal antibodies specific for the human IgG1, IgG2 and IgG3 subclass antibodies were added at a dilution of 1:1000, and IgG4 was added at a 1:500 dilution. The plates with monoclonal antibodies were incubated at 4ºC overnight. Alkaline-phosphatase-labeled goat anti-mouse IgG antibody was added at a 1:2000 dilution for 2 hr at 37ºC. The plates were finally developed with p -nitro phenyl phosphate (alkaline phosphatase substrate). The reaction was stopped with 50 µ l of 3 N NaOH. The color was read at 410 nm in an automated Titerteck plate reader (MR 600; Dynatech). Each incubation was followed by three washes with PBS-T. The antibody activity of test serum pools was expressed as units/ml relative to the antibody activity in the reference serum pool for IgG1, IgG2 and IgG3 antibodies. One unit of antibody activity was equivalent to approximately 0.1 optical density (OD) at 410 nm. The performance characteristics for the reference serum pool have been described in detail previously (11). Since IgG4 antibodies did not show a log distribution across the disease spectrum, sera for IgG4 antibody determination were run at a single dilution of 1:10 and the results of these antibodies are expressed as the OD reading at 410 nm.
Preparation of M. leprae sonicate (MLSON) for SDS-PAGE
Armadillo-derived whole M. leprae (freeze-dried) was provided by Dr. Patrick Brennen (Colorado State University, Fort Collins, Colorado, U.S.A.). M. leprae sonicate was prepared by the following method. Freeze-dried bacilli (20 mg) were suspended in 0.5 ml of distilled water containing a cocktail of protease inhibitors (aprotonin, leupeptin and phenylmethylsulfonyl fluoride). The suspension was vortexed for 15 min. Sonication was carried out on ice for 30 min with a sonifier (Semat Technical, St. Albans, U.K.) using a microprobe with 50 displacement cycles. The sonified bacilli were lyophilizcd and stored at 4ºC until run in SDS-polyacrylamide gel electrophoresis.
Preparation of serum pools for immunoblotting
Serum pools containing high titers of IgG subclass antibodies were prepared from patients with cither lepromatous (N = 12; LL = 9 and BL = 3) or tuberculoid (N = 9; BT = 5 and BT-TT = 4) disease by mixing equal volumes of individual sera. Similarly, a control serum pool was prepared by pooling 10 sera from healthy endemic donors. The mean ± S.D. age of the patients in the three groups were LL/BL, 35.3 ± 3.8; BT/TT, 37 ± 5.7 and endemic controls (EC) 26 ± 2.5. There were no differences in the male-tofemale ratio in the three groups. These pools were clarified by Millipore filtration (0.45 µ m) before use in immunoblotting.
SDS-PAGE
Antigen separation was carried out in Mini Protean II from Biorad according to the procedure described by Laemmli (13). Sample buffer (500 µ l) was added to 20 mg of sonified and freeze-dried preparations of MLSON and boiled for 3 min. MLSON in SDS sample buffer (150 µ l) was applied to a gradient gel (10%-20%) of 1.5-mm thickness. The separation was carried out at 100 volts/20 mA. The sample was allowed to separate until the tracking dye reached within 1.0 cm of the bottom edge of the gel. The separated antigens were stained with either 0.1% solution of Coomassie blue for proteins or with Schiff's reagent for carbohydrates by methods described in detail elsewhere (Pharmacia Fine Chemicals. Polyacrylamide gel electrophoresis, laboratory techniques, 1980, p. 54) or were transferred to nitrocellulose membrane (NCP) for immunoblotting.
Immunoblotting
The separated proteins were electrophoretically transferred by the method of Towbin, et al. (24) to pre-cut NCP (8 x 0.5 cm). The transfer was performed at 10ºC overnight with a constant voltage of 30 volts. The antigen-containing NCP strips were transferred to a slotted incubation tray, and the remaining sites were blocked with PBS-T containing 3% BSA and 1% fetal bovine serum (FBS) for 2 hr at 37ºC. After blocking, the NCP strips were washed with PBS-T and incubated with cither serum pools or individual sera. Lepromatous patients (LL/ BL) were tested at a 1:200 dilution and tuberculoid patients at a 1:20 dilution to adjust for the quantitative differences in antibodies in the two disease groups. The EC pool was tested at both 1:200 and 1:20 dilutions to provide a control for background binding. All sera were diluted in PBS-T and incubated for 2 hr at 37ºC with continuous rocking. After incubation with sera, the NCP strips were incubated with monoclonal antibody probes to the four subclasses of IgG antibodies at a dilution of 1:500 in 1% FBS in PBS-T for 1 hr at 37ºC and then overnight at 4ºC. Again, all monoclonal antibodies were used in excess (12). These strips were subsequently incubated with goat antimouse IgG conjugated to alkaline phosphatase diluted 1:4000 in PBS-T containing 1% FBS for 2 hr at 37ºC and, finally, developed with 5-bromo 4-chloro 3-indolyl phosphate (BCIP)at 1 mg/ml in an amino-methyl-propanol (AMP) buffer. Between each incubation step the NCP strips were washed 4 times (10 min each time) with PBS-T. The color development was stopped after 15 min by rinsing the strips with PBS followed by a final rinse in PBS containing 0.1% sodium azide. The developed strips were photographed when still wet and then dried and stored.
Determination of molecular weight of separated antigens
Molecular weights were assigned to the separated M. leprae antigens using a calibration curve generated by plotting the RF values of the known molecular weight markers separated in the same gel. The intensity of binding was assessed by a scanner (Scan Jet 11; Hewlett Packard, Palo Alto, California, U.S.A.) using a photoshop program on an Apple Macintosh computer. The intensity of the area on the NCP strip without any distinct bands was taken as background and was deducted from the measured intensity while calculating the final intensity. The final intensity was scored as moderate ( + ) or high (++).
RESULTS
IgG subclass antibody activity in serum pools
To assess the differences in antigen recognition in the major types of leprosy (lepromatous versus tuberculoid disease), serum pools from a large panel of patients representative of either disease type were used.
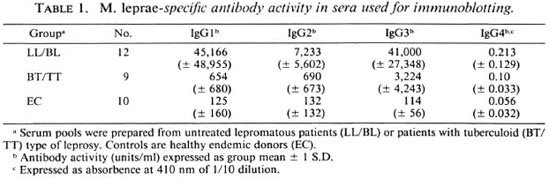
Table 1 shows the antibody activity of individual sera used for the preparation of serum pools. Lepromatous patients showed a 70-fold higher antibody activity for IgG1 and 10-fold higher for IgG2 and IgG3 subclasses when compared to tuberculoid patients. IgG4 antibodies showed some antibody activity in the lepromatous pool but the binding was low (OD < 0.3) even at a dilution of 1:10 as reported previously (11). To adjust for some of these quantitative differences between the two major disease types, we have used lepromatous serum pools at a 10-fold higher dilution (1:200) than tuberculoid serum pools (1:20) for all four IgG subclass antibodies in the immunoblotting assays. The sensitivity of detection in the ELISA is 1 unit/ml. Therefore, we feel that all IgG subclass antibodies were several-fold in excess of their detection limit in the immunoblotting analysis since the principle of the test and the reagents used were identical in the two assays.
Recognition of M. leprae antigens by IgG subclasses
Identification of protein and carbohydrate components in M. leprae.
The carbohydrate and protein staining pattern of M. leprae sonicate separated on a 10%-20% polyacrylamide gradient gel is shown in Figure 1. The carbohydrate staining pattern (periodic acid Schiff) resulted in three distinct but diffused bands of molecular weights of 28-39 kDa, 18-25 kDa and 2-9 kDa, with the strongest staining observed with 2-9 kDa. More than 26 distinct protein bands were present in the M. leprae sonicate preparation, ranging in molecular weight from 14-100 kDa (Fig. 1). All protein and carbohydrate bands were effectively transferred to NCP as determined by the staining of the gels after transfer as well as the staining of the NCP strips (data not shown). Immunoblotting was then carried out to identify which of these bands were recognized by individual IgG subclass antibodies.
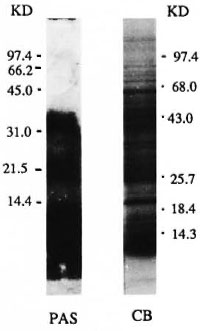
Fig. 1. Protein and carbohydrate staining of M. leprae sonicate. The antigens were separated on a Polyacrylamide gradient (10%-20%). Lane 1 = pattern after periodic acid Schiff (PAS) staining and Lane 2 = pattern of M. leprae sonciate after Coomassie blue (CB) staining. Numbers indicate the molecular weights in kilodaltons of the markers used.
Immunoblotting analysis. In addition to the serum pools representing the lepromatous and tuberculoid forms of the disease, one patient representing each of the disease subtypes (LL, BL, BT, TT) with high titers of antibody was also analyzed to sec if differences also exist at the disease subtype level.
Lepromatous and tuberculoid patients showed a distinct pattern of antigen recognition by each of the four IgG subclass antibodies in serum pools (Fig. 2). IgG1 antibody binding in the serum pool from LL/ BL patients resulted in several sharp bands (lane 1), while a more diffuse IgG1 antibody-binding pattern was obtained with the serum pool from BT/TT patients (lane 5), indicating that in tuberculoid leprosy patients IgG1 antibodies may be recognizing either heavily glycosylated proteins or recognizing both carbohydrate and protein moeities. The diffuse pattern with IgGl antibody in tuberculoid patients was not due to higher background binding, since IgGl antibody showed only a few weak bands with the control (EC) serum pool at 1:20 (lane 8). IgG1 antibody binding observed with the tuberculoid serum pool was distinct from that observed with the control EC pool, indicating that the recognition in tuberculoid patients was related to the disease. Although only one patient was tested from each lepromatous disease subtype (LL and BL), it was interesting to note that IgG1 antibody recognition was different for these individual LL and BL patients, indicating that differences may exist even at the disease subtype level. Since there was a very weak signal with IgGl antibody in the TT patients above the control (EC) scrum pool, differences between BT and TT patients could not be evaluated.
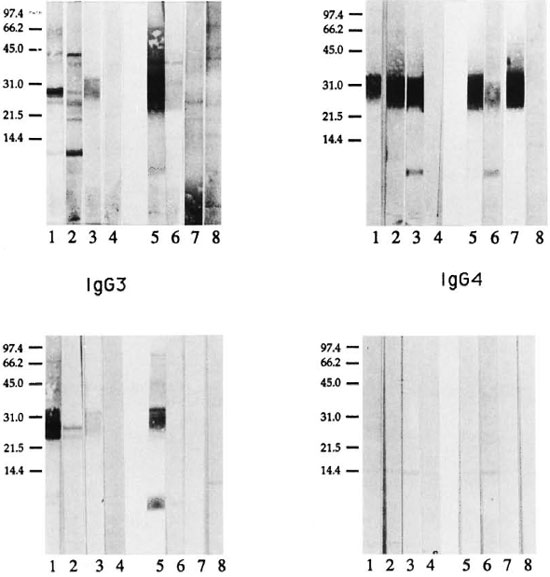
Fig. 2. IgG subclass recognition of it, leprae antigens in patients across the leprosy spectrum. Immunoblot analysis was carried out using mouse monoclonal antibodies specific for human IgG subclass antibodies and enzyme-linked anti-mouse IgG antibody as probes. Lepromatous patient (LL/BL) sera were tested at 1:200 and tuberculoid patient (BT/TT) sera were tested at 1:20. Lane 1 = LL/BL serum pool; lane 2 = LL scrum; lane 3 = BL serum; lane 4 = endemic control serum pool (1:200); lane 5 = BT/TT serum pool; lane 6 = BT serum; lane 7 = TT serum; lane 8 = endemic control serum pool (1:20).
IgG2 antibody recognition was similar in the two disease groups (Fig. 2, lanes 1 and 5) with both disease groups strongly recognizing a restricted region corresponding to one of the carbohydrate-staining regions at 28-39 kDa. The molecular weight of this region corresponds to lipoarabinomannan (LAM) described in M. leprae (4). This binding is highly specific since little or no background binding was observed with control sera at either a 1:200 or a 1:20 dilution (Fig. 2, lanes 4 and 8). Surprisingly, the 2-9 kDa region, which stained strongly for carbohydrates only (Fig. 1), was not recognized by either the LL/BL or the BT/TT serum pools. However, a sharp band was seen at 5-7 kDa by individual BL (Fig. 2, lane 3) and BT patients (Fig. 2; lane 6), indicating that IgG2 antibodies to this band may have been diluted in the serum pools. Secondly, differences also may exist at the disease subtype level and need to be evaluated with a larger panel of patients in each disease subtype.
IgG3 antibody subclass recognition in the lepromatous serum pool was more restricted than IgGl antibodies. IgG3 antibodies showed mostly diffused bands in the region of 25-36 kDa in both lepromatous (Fig. 2, lane l)and tuberculoid patients (Fig. 2, lane 5). In addition, tuberculoid patients showed strong recognition of 5-7 kDa, while lepromatous patients showed only a weak binding at the 6 and 8 kDa. Very little background binding was observed for IgG3 antibody in control scrum at cither a 1:200 or a 1:20 dilution. Among the subtypes of lepromatous patients (LL, BL), LL patients showed IgG3 antibody binding to two discrete bands at 25 and 28 kDa while BL patients showed a weak and diffuse pattern of binding between the 25-36 kDa region. The issue of differences in IgG subclass antibody recognition among the leprosy disease subtypes, therefore, needs to addressed more rigorously.
IgG4 antibody recognition, which is linked to Th2 activation, showed very weak binding to a single band at 28 kDa, and only in the LL/BL pool. One distinct band was also observed with IgG4 at 15 kDa in both leprosy and control sera, and may not be disease related.
Dominant M. leprae antigens recognized by IgG subclasses in polar leprosy groups
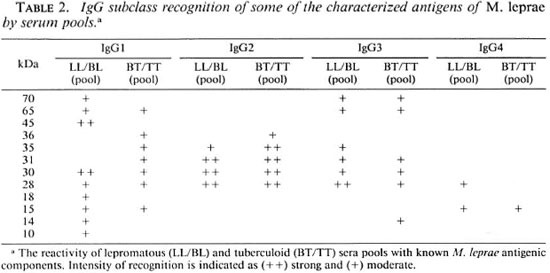
The molecular weights of the known antigens of M. leprae (27) and the intensity of binding by each of the four IgG subclass antibodies in the serum pools is given in Table 2. IgG1 antibody in the lepromatous scrum pool recognized a majority of known M. leprae protein antigens, including a number of heat-shock proteins (70, 65, 45, 30, 28, 18, 15, 10 kDa). IgG1 antibody in tuberculoid patients also recognized known antigens 65, 36 and 15 kDa. IgG2 antibody binding was observed to a carbohydratestaining region between 25-36 kDa which corresponds to the M. leprae LAM (4) and was similar for both lepromatous and tuberculoid serum pools. Although IgG3 recognized a much more restricted set of protein antigens than IgGl, most of the antigens recognized were known antigens (70, 65, 35, 31, 30 and 28). IgG4 subclass antibody in lepromatous patients showed weak binding to a 28-kDa protein which has been shown to have superoxide dismutase activity (23).
DISCUSSION
T cells are differentially activated across the leprosy spectrum, with the highest T-cell activity at the tuberculoid end and the lowest toward the lepromatous pole of the disease (8). Switching of IgM antibodies to one of the four IgG subclasses is regulated by cytokines secreted from activated T cells. However, little is known about the effect of differential T-cell activation in leprosy on IgG subclass antibody response. Three of the four IgG subclass antibodies to M. leprae (IgG1, IgG2 and IgG3) previously have been reported to be present across the disease spectrum irrespective of the level of T-ccll activation (7,11). The only differences observed across the disease spectrum were at the quantitative levels (11).
In the current study when IgG subclass antibody recognition was dissected at the qualitative level, differences in the antigen recognition patterns across the leprosy spectrum became apparent. The major difference in the IgG subclass antigen recognition pattern was observed with IgG1 and IgG3 antibodies in the two polar groups of leprosy patients. Both IgG1 and IgG3 antibodies are known to recognize protein antigens which require T-cell-derived factors for switching and maturation of the immune response (22) and may well be reflected in the different sets of antigens being recognized by lepromatous and tuberculoid patients. Differences were noted even among the subtypes of lepromatous patients (lepromatous, LL and borderline lepromatous, BL). Although only one individual patient was analyzed in the current study, LL and BL patients showed differences for both IgG1 and IgG3 subclass antibodies. This may well be due to differences in the T-cell activity in LL and BL patients since in BL patients some T-cell activity is reported (16). This is further supported by the observation that BL patients showed antigen recognition patterns which had more similarity with BT patients than with LL patients. This issue needs to be confirmed further with a large panel of individual patients in the subtypes of both lepromatous and tuberculoid groups. The IgG2 antibody response, which is known to be a T-independent response, was similar in both disease types irrespective of T-cell activation. IgG4 antibody responses are dependent on cytokines elaborated by the Th2 subset. These Th2 cytokines have been shown to be upregulated in skin lesions of lepromatous but not of tuberculoid patients (19,26). IgG4 antibody showed weak binding to one antigen (28 kDa) in the lepromatous (LL/BL) serum pool only. Our results suggest that selective activation of a T-cell subset at local disease sites may not necessarily be reflected in the blood compartment due to dilution effects.
As reported previously, carbohydrate antigens, particularly lipoarabinomman (LAM, 30-40 kDa), evoked strong IgG2 antibody responses in both lepromatous and tuberculoid patients (7). Since type 1 carbohydrate antigens arc T-independent antigens, this finding was not unexpected. What was surprising was that not all carbohydratestaining regions evoked IgG2 antibody responses. Surprisingly, the strongest carbohydrate staining region (2-9 kDa) which was not stained by Coomassie blue was recognized by IgG3 antibodies but not by IgG2 antibodies in tuberculoid leprosy patients, suggesting that certain molecular constraints may be present in the recognition of carbohydrate antigens by IgG2 subclass antibodies. Since the region recognized by IgG3 is either carbohydrate or glycolipid in nature, it is therefore distinct from the low molecular weight polypeptides described by Andersen and Heron (1) in the same region for M. tuberculosis, which evoke strong delayed-type hypersensitivity (DTH) and T-cell memory responses in guinea pigs (1). Recently a new subset of T cells (CD1 + CD4-CD8) has been described (17) which recognizes lipid (3) and lipoglycan antigens (20) in association with CD1 molecules possessing cytolytic activity. This antigen may well be a candidate antigen for CD1 T cells.
In tuberculoid leprosy patients, IgG1, IgG2 and IgG3 antibodies all resulted in a diffuse band in the region of 25-36 kDa. It is unlikely that LAM (30-40 kDa) is inducing all three IgG subclass antibodies since purified LAM has been shown to bind to only IgG2 antibodies (6). One possible explanation for the differences in antigen recognition by IgGl and IgG3 antibodies in tuberculoid and lepromatous patients may be the differential processing and clearance of antigen in the two groups. In tuberculoid patients, cell-wall-associated antigens may be released due to the killing of M. leprae by activated macrophages because of the presence of activated T cells (6). These antigens may then subsequently induce antibody responses.
In lepromatous patients, a larger number of secreted antigens would be available due to the higher viable bacterial load inducing responses primarily to these antigens and, hence, the differences in the immune response profile. The predominant recognition of carbohydrate antigens in tuberculoid patients would not be unexpected since carbohydrate antigens arc less degradable and tend to remain in the tissues much longer; whereas protein antigens would be cleared much more rapidly. Because of the strong recognition of LAM by IgG2 antibodies across the disease spectrum, this response could be exploited for diagnostic purposes. A clear understanding of the relationship between the IgG subclass response and the T-ccll activation would allow a better understanding of disease progression in leprosy and, also, would help in identifying antigens which may be important in the protective response.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
We would like to thank Drs. Thomas J. Chiang and Qadccr Ahsan at Marie Adelaide Leprosy Centre, Karachi, Pakistan, for their help in obtaining clinical material. We also want to thank Dr. Sebastian Lucas, The Middlesex Hospital, London, for performing the histopathology. Excellent technical assistance was provided by Miss Maqboola Dojki and Miss Nabila Abrar in the separation and storage of sera and the performing of the assays. Secretarial help in manuscript preparation was provided by Miss Regina Paul.
REFERENCES
1. ANDERSEN, P. and HERON, I. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect. Immun. 61(1993)844-851.
2. AUGENER, W., GREY, H. M., COOPER, N. R. and MULLER-EBERHARD, H. J. The reaction of monomelic and aggregated immunoglobulins with C1. Immunochemistry 8(1971)1011-1020.
3. BECKMAN, E. M., PORCELLI, S. A., MORITA, C. T., BEHAR, S. M., FURLONG, S. T. and BRENNER, M. B. Recognition of lipid antigen by CD 1 -restricted αβ + T cells. Nature 372(1995)691-694.
4. CHATTERJEE, D., HUNTER, S. W., MCNEIL, M. and BRENNAN, P. J. Lipoarabinomannan; multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J. Biol. Chem. 267 (1992) 6228-6233.
5. CLOSS, O., MSHANA, R. N. and HARBOE, M. Antigenic analysis of Mycobacterium leprae. Scand. J. Immunol. 9(1992)297-302.
6. DA COSTA, C. T. K. A., KHANOLKAR-YOUNG, S., ELLIOT, A. M., WASUNNA, K. M. A. and MCADAM, K. P. W. J. Immunoglobulin G subclass response to mycobacterial lipoarabinomannan in HIV-infected and noninfected patients with tuberculosis. Clin. Exp. Immunol. 91(1993) 25-29.
7. DHANDAYUTHAPANI, S., IZUMI, S., ANANDAN, D. and BHATIA, V. N. Specificity of IgG subclass antibodies in different clinical manifestations of leprosy. Clin. Exp. Immunol. 88(1992)253-257.
8. HARBOE, M. The immunology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, Ltd., 1985, pp. 53-87.
9. HUBER, H., DOUGLAS, S. D., NUSBACHER, J., KOCHWA, S. and ROSENFIELD, R. E. IgG subclass specificity of human monocytes receptor sites. Nature 229(1971)419-420.
10. HUSSAIN, R., DOCKRELL, H. M. and CHIANG, T. J. IgG subclass antibody to Mycobacterium leprae 18000 MW antigen is restricted to IgGl and lgG3 in leprosy. Immunology 83(1994)495-500.
11. HUSSAIN, R., KIFAYET, A. and CHIANG, T. J. IgGl and IgG3 are the markers of progressive disease in leprosy. Infect. Immun. 63 (1994) 410-415.
12. HUSSAIN, R., POINDEXTER, R. W., OTTESEN, E. A. and REIMER, C. B. Use of monoclonal antibodies to quantify subclasses of human IgG. II. Enzyme immunoassay to define antigen specific (anti-filarial) IgG subclass antibodies. J. Immunol. Mcth. 94(1986)73-80.
13. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227(1970)680-685.
14. MELSOM, R., HARBOE, M., MYRVANG, B. and GODAL, T. Immunoglobulin class specific antibodies to M. leprae in leprosy patients including the indeterminate group and healthy contacts as a step in the development of methods for serodiagnosis of leprosy. Clin. Exp. Immunol. 47(1982)225-233.
15. MOSMANN, T. R., CHERWINSKI, H., BOND, M. W., GIEDLIN, M. A. and COFFMAN, R. L. TWO types of helper T-cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136(1986)2348-2357.
16. MYRVANG, B., GODAL, T., RIDLEY, D. S., FRO-LAND, S. S. and SONG, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14(1973)541-553.
17. PORCELLI, S., MORITA, C. T. and BRENNER, M. B. CD 1 b restricts the response of human CD4 CDS T-lymphocytes to a microbial antigen. Nature. 360(1992) 593-597.
18. RIDLEY, D. S. Histological classification and immunological spectrum of leprosy. Bull. WHO 51(1974)451-464.
19. SALGAME, P., YAMAMURA, M., BLOOM, B. R. and MODLIN, R. L. Evidence of functional subsets of CD4' and CD8* T cells in human disease: lymphokine patterns in leprosy. Chem. Immunol. 54(1992) 44-59.
20. SIELING, P. A., CHATTERJEE, D., PORCELLI, S. A., PRIGOZY, T. L, SORIANO, T., BRENNER, M. B., KRONENBERG, M., BRENNEN, P. J. and MODLIN, R. L. CD1 restricted T cell recognition of microbial lipoglycans. Science (1995) in press.
21. SNAPPER, C. M. The cellular and molecular biology of cytokine directed murine IgG isotype production. In: The Human IgG Subclasses: Molecular Analysis of Structure, Function and Regulation. Shakib, F.,cd. London: Pcrgamon Press, 1990, pp. 251-274.
22. SNAPPER, C. M. and FINKELMAN, F. D. Immunoglobulin class switching. In: Fundamental Immunology. 3rd cdn. Paul, W. E., ed. New York: Raven Press Ltd., 1993, pp. 837-863.
23. THANGARAJ, H. S., LAMB, F. I., DAVIS, E. O. and COLSTON, M. J. Nucleotide and deduced amino acid sequence of M. leprae manganese superoxide dismutasc. Nucl. Acid Res. 17(1989)83-87.
24. TOWBIN, H., STAEHAELIA, T. and GODON, G. Elcctrophorctic transfer of proteins from polyacrylamidc gels to nitrocellulose sheets; procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76(1979)4350-4354.
25. WRIGHT, E. P., VLUG, A., GEERTZEN, H. G. M., LONG, H. T. and HONG, N. D. Serum immunoglobulins including IgG subclasses in Vietnamese leprosy patients. Int. J. Up . 53(1985)225-232.
26. YAMAMURA, M., UYEMURA, K., DEANS, R. J., WEINBERG, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277-279.
27. YOUNG, D. B., KAUFMANN, S. H. E. and HERMANS, P. W. M. Mycobacterial protein antigens: a compilation. Mol. Microbiol. 6(1992) 133-145.
1. M.Sc; Department of Microbiology, The Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 74800, Pakistan. Tel=92-21-4930051, ext. 2145.
2. Ph.D., M.R.C. (Path.), Department of Microbiology, The Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 74800, Pakistan. Tel=92-21-4930051, ext. 2145.
Reprint requests to Dr. Hussain at above address or FAX 92-21-4934294.
Received for publication on 2 May 1995;
Accepted for publication in revised form on 19 October 1995.