- Volume 64 , Number 2
- Page: 105–14
Selective decrease of M. leprae-specific lgG1 and lgG3 antibodies in leprosy patients associated with ENL
ABSTRACT
Erythema nodosum leprosum (ENL) is a serious complication of lepromatous leprosy. Because of the similarities with the Arthus-type reaction, ENL is presumed to be due to immune complex formation and their deposition in the tissues. The aim of this study was to dissect the antibody response at the IgG subclass level to ascertain differences in IgG subclasses in nonecactional lepromatous/borderline lepromatous (LL/BL) patients and reactional (ENL) lepromatous patients. The ENL group showed significantly lower serum antibody levels for the four subclasses compared to the LL/BL group of patients using the Mann-Whitney U test (IgG 1, p = 0.0001; IgG2, p = 0.0009; IgG3, p = 0.0001; IgG4, p = 0.03). Since the majority of ENL patients (54 of 67) had received leprosy chemotherapy for varying durations of time, LL/BL patients were also compared with 19 ENL patients who had received <2 weeks of chemotherapy. In this group only IgGl (p = 0.048) and IgG2 (p = 0.001) antibodies showed significantly lower concentrations. Immunoblotting analysis demonstrated that in ENL patients IgGl showed a selective disappearance of several antigenic bands recognized by the LL/BL serum pool; while most of the antigens recognized by IgG3 antibodies in the LL/BL serum pool were not detected in the ENL serum pool or in the sera of pretreated individual ENL patients. These results suggest that IgG 1 and IgG3 may be the most pathogenic IgG subclass antibodies during ENL, and their deposition in tissues could initiate the complement-mediated inflammatory pathway resulting in the clinical disease associated with ENL.RÉSUMÉ
L'érythèmc noueux lépreux (ENL) est une complication sérieuse de la lèpre. A cause de ses similitudes avec la réaction de type Arthus, l'ENL est supposé être due à la formation de complexes immuns et à leur dépôt dans les tissus. Le but de celte étude était d'examiner la réponse en anticorps au niveau de la sousclasse IgG pour évaluer les différences dans les sousclasses IgG chez les patients lépromateux et borderline lépromalcux non-réactionnels (LL/BL) et des patients lépromateux réactionnels (ENL). Le groupe ENL a montré, par le test U de Mann-Whitney, des taux d'anticorps sériques significativement plus bas pour les quatre sous-classes, comparé au groupe LL/BL de patients (IgGl, p = 0.0001; IgG2, p = 0.0009; IgG3, p = 0.0001; IgG4, p = 0.03). Puisque la majorité des patients ENL (54 sur 67) avait suivi une chimiothérapie anti-lépreuse pour des durées variables, les patients LL/BL ont aussi été comparés aux 19 patients ENL qui avaient reçu < 2 semaines de chimiothérapie. Dans ce groupe, seuls les anticorps IgGl (p = 0.048) et IgG2 (p = 0.001 ) montraient des concentrations significativement plus faibles. Une analyse par immunoblottinga montré que chez les patients ENL les IgGl montraient une disparition sélective de diverses bandes antigéniques reconnues par le pool des serums LL/BL; tandis que la majorité des antigènents ENL individuels en avant traitement. s reconnus par les anticorps IgG3 du pool de serums LL/BL n'était pas détectée dans le pool des serums ENL ni dans les serums des patieCes résultats suggèrent que les IgGl et IgG3 pourraient être la sous-classe d'anticorps la plus pathogène durant l'ENL, et leur dépôt dans les tissus pourrait déclencher le processus inflammatoire dans lequel intervient le complément résultant dans la maladie clinique associée avec l'ENL.RESUMEN
>El eritema nodoso leproso (ENL) es una seria complicación de la lepra lepromatosa. Debido a su similitud con la reacción de Arthus, se asume que el ENL se debe a la formación de complejos inmunes y su depósito en los tejidos. El objeto de este estudio fue el analizar la respuesta en anticuerpos a nivel de las subclases de IgG para establecer las posibles diferencias entre los pacientes LL/BL reaccionales y no reaccionales. Usando la prueba de U de Mann-Whitney, el grupo ENL mostró niveles de anticuerpos de las 4 subclases de IgG significativamente menores que los pacientes LL/BL sin reacción (IgGl, p = 0.0001; IgG2, p = 0.0009; IgG3, p = 0.0001; IgG4, p = 0.03). Puesto que la gran mayoría de los pacientes ENL (54 de 67) habian recibido quimioterapia por periodos variables de tiempo, los pacientes LL/BL también se compararon con 19 pacientes que solo habían recibido tratamiento por menos de 2 semanas. En este grupo, sólo los anticuerpos IgGl (p = 0.048) e IgG2 (p = 0.001) mostraron concentraciones significativamente mas bajas. Los análisis por ";western blot"; demostraron que los anticuerpos IgGl del suero de los pacientes con ENL reconocieron menos componentes antigénicos que los anticuerpos de una mezcla de sueros LL/BL, y que la mayoría de los antígenos reconocidos por los anticuerpos IgG3 de suero BL/LL no fueron detectados por los sueros de los pacientes con ENL tratados o no tratados. Los resultados sugieren que los anticuerpos IgG 1 e IgG3 pueden ser las subclases más patogenéticas de IgG durante el ENL, y que su depósito en los tejidos podria iniciar la activación de complemento y la inflamación característica del cuadro reaccional.In patients with leprosy, erythema nodosum leprosum (ENL) occurring toward the lepromatous pole of the disease is one of the most serious reactional complications which can result in permanent disability. Nearly 50% of the patients with lepromatous disease develop ENL by the end of their first year of chemotherapy (). Clinically, ENL is characterized by recurrent crops of painful, red, indurated subcutaneous nodules arising in apparently normal skin which may become necrotic, pustular and hemorrahagic. Patients with severe ENL can develop fever, lymphadenopathy, albuminuria and arthritis as well as iridocyclitis, orchitis and neuritis (15). Most of the clinical and histological features associated with ENL resemble serum sickness (23). The demonstration of immune complexes in the serum (4-16) and in the skin (20, 23) adds weight to the immune complex pathogenesis of ENL. The factors which precipitate ENL reactions in lepromatous patients are still not well understood. Although patients with lepromatous leprosy show high concentrations of acid-fast bacilli (AFB) in tissues as well as antibodies of both IgM and IgG isotypes in the circulation (14), patients undergoing ENL tend to have significantly lower concentrations of both IgM and IgG anti- Mycobacterium leprae antibodies compared to nonreactional lepromatous patients (1,10). Since ENL reactions are occurring in tissues, extravasation of IgG antibodies is more likely to occur than of any other isotype. Human immunoglobulin G is composed of four distinct subclasses which differ in their structure, concentration and biological activities such as complement fixation ( 26 ) and binding to cell surface receptors on monocytes/macrophages (7).
We have recently reported that in leprosy the most prominent response to M. leprae antigens was observed with IgGl and IgG3 antibodies (8-9), which were not only disproportionally elevated but also showed the highest correlation with the bacterial load across the leprosy spectrum (9). IgG3 and IgG 1 subclasses are the most highly efficient antibodies in fixing complement (2-3). During antigen clearance the presence of such high proportions of complement-fixing antibodies in parallel with high antigenic load may lead to pathogenic consequences by deposition in tissues and by release of inflammatory mediators resulting in clinical symptoms associated with ENL episodes.
To address this issue we have further dissected the IgG subclass antibody response to M. leprae in patients undergoing ENL at the quantitative level, using ELISA, and at the qualitative level, using immunoblotting methodology. Our results demonstrate that only IgGl and IgG3 antibodies were significantly lower in lepromatous patients undergoing ENL reaction compared to nonreactional leprosy patients with lepromatous disease.
MATERIALS AND METHODS
Patients and controls. Leprosy patients were recruited at the Marie Adelaide Leprosy Center in Karachi, Pakistan. A 4-mm punch biopsy was taken from a representative skin lesion and fixed by conventional formol-mercuric chloride-acetic acid fixative (FMA), processed to paraffin, and stained with hematoxylin and eosin (H&E) and Wade-Fite stains for AFB. Leprosy lesions were categorized using standard clinical and histopathological features (18,19) . Sixty-seven lepromatous patients undergoing acute ENL (61 LL and 6 BL) were included in the study based on their clinical signs. Only 70% of ENL patients showed typical histological features of ENL. Typical ENL was recognized as the classical ";pink node"; type (18), with a polymorphonuclear neutrophil (PMN) infiltrate (with/without vasculitis) into the dermis and subcutaneous tissues of lepromatous lesions. ENL patients with clinical signs and symptoms who could not be confirmed histologically were also included in the study based on our previous observation that these patients have high concentrations of acute phase proteins which are inflammatory markers and, therefore, are undergoing ENL reactions (l0). Control groups included: 1) 83 nonreactional patients with lepromatous disease who had received chemotherapy for leprosy for < 2 weeks. Lepromatous patients were confirmed histologically as either polar lepromatous (LL; N = 44) or borderline Jepromatous (BL; N = 39); and 2) 77 healthy endemic controls without known contact with leprosy who were employed at The Aga Khan University Medical Center.
Serum. Five milliliters of peripheral blood was obtained from each of the patients and control subjects. The blood was allowed to clot overnight at 4ºC. Serum was removed and centrifuged at 400 x g x 15 min; the clear supernate was distributed in 100 µl aliquots and kept at - 70ºC until use.
Reagents. Monoclonal antibodies specific for human IgG subclasses were HP6069 (anti-IgGl) kindly provided by Dr. Hamilton, John Hopkins University, Baltimore, Maryland, U.S.A.; HP 6002 (anti-IgG2), HP 6047 (anti-IgG3), HP 6023 (anti-IgG4), prepared at the Centers for the Disease Control, Atlanta, Georgia, U.S.A., were a gift from Dr. Reimer. Goat anti-mouse IgG conjugated to alkaline phosphatase was purchased from Jackson Immuno Research Laboratories, West Grove, Pennsylvania, U.S.A., and was used according to manufacturer's recommendations. Bovine serum albumin (BSA), 5-bromo 4-chloro 3-indolyl phosphate (BCIP), p -nitro-phenyl phosphate, β-mercaptoethanol and Tris hydroxy methyl amino methane were obtained from Sigma Chemical Co., St. Louis, Missouri, U.S.A. Acrylamide, bis-acrylamide, sodium dodecyl sulfate (SDS), glycine, ammonium persulfate, Coomassie blue and nitrocellulose membrane (0.45-µm) were purchased from Bio-Rad, Richmond, California U.S.A.
Quantitative determination of M. leprae specific IgG subclasses. ELISAs were carried out on 96-well, flat-bottom, polystyrene Immulon 4 plates (Dynatech, McLean, Virginia, U.S.A.) as described in detail previously (9). Briefly, M. leprae soluble sonicate (batch CD114 kindly provided by Dr. R. J. W. Rees, National Institute of Medical Research, London, U.K.) was coated at 0.4 /µx/well in 100 /µ\ of carbonate buffer at pH 9.6, incubated for 2 hr at 37ºC and additionally incubated at 4ºC overnight. The plates were washed with phosphate buffered saline containing 0.05% Tween 20 (PBS-T) and the remaining binding sites were blocked with 5% BSA in PBS at 37ºC for 2 hr. Reference and test sera were diluted 1/1000 in PBS-T containing 1% BSA. These diluted sera were then added to the wells at twofold serial dilutions.
All test sera were run at a minimum of four dilutions. The sera were incubated for 2 hr at 37ºC followed by an overnight incubation at 4ºC. Monoclonal antibodies specific for the human IgG 1, IgG2 and IgG3 subclasses were added at a dilution of 1:1000 and that for IgG4 was added at a 1:500 dilution. The plates with monoclonal antibodies were incubated at 4ºC overnight. Alkaline-phosphatase labeled goat anti-mouse IgG antibody was added at a 1:2000 dilution for 2 hr at 37ºC. The plates were finally developed with p-nitro phenyl phosphate (alkaline phosphatase substrate). The reaction was stopped with 50µl of 3 N NaOH. The color was read at 410 mm in an automated Titertek plate reader MR 600 (Dynatech). Each incubation was followed by three washes with PBS-T. The antibody activity of test sera was expressed as units/ml. These units were calculated from a reference curve (for reference serum pool) generated in units relative to optical density (OD). The performance characteristics for the reference serum pool have been described in detail previously (9). The limit of detection for IgGl, IgG2 and IgG3 antibodies was one unit equivalent to ~0.1 OD. Thus, a serum with 34, 800 units is equivalent to 0.1 OD at a 1/34,800 dilution. Since IgG4 antibodies did not show a log distribution across the disease spectrum, sera for IgG4 antibody determination were run at a single dilution of 1:10 and the results of these antibodies are expressed as OD reading at 410 nm.
Preparation of M. leprae sonicate (MLSON) for SDS-PAGE. Armadillo-derived whole M. leprae (freeze-dried) was provided by Dr. Patrick Brennen (Colorado State University, Fort Collins, Colorado, U.S.A.). M. leprae sonicate was prepared by the following method. Freeze-dried bacilli (20 mg) were suspended in 0.5 ml of distilled water containing a cocktail of protease inhibitors [aprotonin, leupeptin and phenylmethylsulfonyl fluoride (PMSF)]. The suspension was vortexed for 15 min. Sonication was carried out on ice for 30 min with a sonifier (Semat Technical, St. Albans, U.K.) using a microprobe with 50 displacement cycles. The sonified bacilli were lypholized and stored at 4ºC until run in SDSpolyacrylamide gel electrophoresis.
Preparation of serum pools for immunoblotting. Serum pools containing high titers of IgG subclass antibodies were prepared from 12 leprosy patients with either nonreactional lepromatous disease (9 LL and 3 BL) or 23 lepromatous patients undergoing ENL reaction (21 LL and 2 BL) by mixing equal volumes of individual sera. Similarly, a control serum pool was prepared by pooling 10 sera from healthy endemic donors. The mean ages of the patients in the three groups were: LL/BL, 35.3 ± 3.8; ENL, 35.6 ± 16.6 and endemic controls 26 ± 2.5. There was no difference in the male/female ratio in the three groups. These pools were clarified by Millipore filtration
(0.45 /µm) before use in immunoblotting. SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Antigen separation was carried out in Mini Protean II from Bio-Rad according to the procedure described by Laemmli (12). Sample buffer (500 µl) was added to 20 mg of sonified preparation of MLSON and boiled for 3 min. MLSON in SDS sample buffer (150µl was applied to a gradient gel (10%-20%) of 1.5-mm thickness. The separation was carried out at a constant current of 20 mA. The sample was allowed to separate until the tracking dye reached within 1.0 cm. of the bottom edge of the gel. The separated antigens were either stained with a 0.1% solution of Coomassie blue for proteins or were transferred to a nitrocellulose membrane (NCP) for immunoblotting.
Immunoblotting. The separated proteins were electrophoretically transferred by the method of Towbin, et al. (22) to pre-cut NCP (8 x 0.5 cm). Transfer was performed at 10ºC overnight with a constant voltage of 30 volts. The antigen-containing NCP strips were transferred to a slotted incubation tray, and the remaining sites were blocked with PBS-T containing 3% BSA and 1% fetal bovine serum (FBS) for 2 hr at 37ºC. After blocking, the NCP strips were washed with PBS-T and incubated with either serum pools or individual sera at a 1:200 dilution. All sera were diluted in PBS-T and incubated for 2 hr at 37ºC with continuous rocking. After incubation with sera, the NCP strips were incubated with monoclonal antibody probes to the four subclasses of IgG antibodies at a dilution of 1:500 in 1% FBS in PBS-T for 1 hr at 37ºC and then overnight at 4ºC. These strips were subsequently incubated with goat anti-mouse IgG conjugated to alkaline phosphatase diluted 1:4000 in PBS-T containing 1% FBS for 2 hr at 37ºC, and finally developed with 5-bromo 4-chloro 3-indolyl phosphate (BCIP) at 1 mg/ml in amino-methyl-propanol (AMP) buffer. Between each incubation step the NCP strips were washed 4 times (10 min each time) with PBS-T. The color development was stopped after 15 min by rinsing the strips with PBS followed by a final rinse in PBS containing 0.1% sodium azide. The developed strips were photographed when still wet. The presence and intensity of the antigenic bands on each NCP strip was assessed visually and was scored as strong (+) or weak (±). Molecular weights of these bands were calculated using a calibration curve generated by plotting the RF values of the known molecular weight markers separated in the same gel.
Statistical analysis. Descriptive analysis including geometric means and standard deviations were carried out on a Macintosh Plus microcomputer using StatviewTM software packages and microsoft ExcelTM packages. Nonparametric analysis (Mann-Whitney U test) was carried out to assess the significance of difference between the antibody levels in the different patient groups.
RESULTS
Quantitation of IgG subclass responses in patients with ENL. To understand the role of IgG subclasses during acute ENL, concentrations of IgG subclass antibodies to M. leprae were determined in lepromatous patients undergoing acute ENL reaction and compared to nonreactional leprosy patients with lepromatous disease (LL/BL). Figure 1 shows the distribution of antibody activity for the four subclasses of IgG (IgGl, IgG2, IgG3 and IgG4). All four IgG subclass antibodies showed significantly lower concentrations (IgGl, p < 0.0001; IgG2, p = 0.0009; IgG3, p < 0.0001; IgG4, p = 0.03) in patients undergoing ENL reaction compared to nonreactional LL/BL patients.
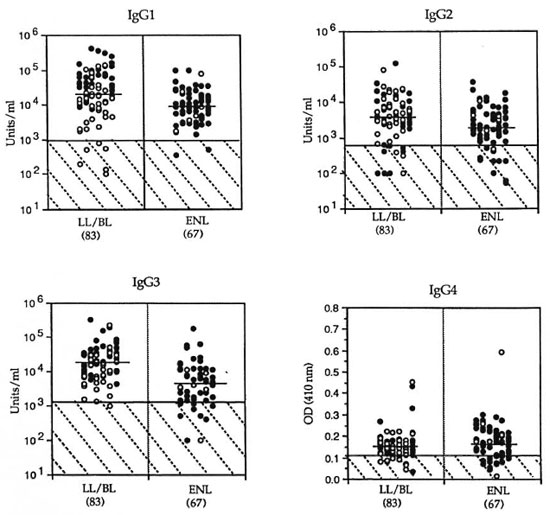
Fig. 1. IgG subclass antibodies to M.leprae -soluble antigens in nonreactional lepromatous (LL/BL) patients and patients with erythema nodosum leprosum (ENL) reaction.  = LL patients;
= LL patients;  = BL patients. Number in each group is indicated in parentheses. Antibody activity for IgGl, IgG2 and IgG3 is given as units/ml; for IgG4, as optical density measurement at a serum dilution of 1:10. Horizontal bars = geometric mean for each group. Hatched area indicates group mean of endemic population + 2 S.D.
= BL patients. Number in each group is indicated in parentheses. Antibody activity for IgGl, IgG2 and IgG3 is given as units/ml; for IgG4, as optical density measurement at a serum dilution of 1:10. Horizontal bars = geometric mean for each group. Hatched area indicates group mean of endemic population + 2 S.D.
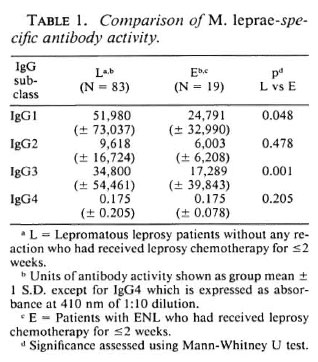
A majority of ENL patients (54 of 67) in the study had received chemotherapy for leprosy of varying durations (1-60 months). Chemotherapy also results in a decrease of IgG antibody levels (5-13). Therefore, it may be inappropriate to use nonreactional LL/ BL patients who had received leprosy chemotherapy for 0-2 weeks for comparison with ENL patients who had been treated long term for leprosy. Of the 67 ENL patients, 28% (19 of 67) had received chemotherapy for leprosy for <2 weeks. We, therefore, compared this group with the nonreactional LL/BL group (Table 1). Interestingly, in this group of ENL patients only IgGl and IgG3 antibodies showed significantly lower concentrations (IgGl, p = 0.048; IgG3, p = 0.001), indicating that the decrease in the other two IgG subclass antibodies (IgG2, IgG4) may be related to chemotherapy while the lower concentrations of IgGl and IgG3 antibodies may, in fact, be due to the ongoing ENL reaction.
Recognition of M. leprae antigens by IgGl and IgG3 subclass antibodies in patients with ENL. It is also important to identify M. leprae antigens which evoke potent IgGl and IgG3 subclass antibody responses and which may be actively forming immune complexes and depositing in the tissues during ENL. Serum pools from patients with either nonreactional lepromatous disease (LL/BL) or with acute ENL reaction were analyzed by immunoblotting analyses (Fig. 2). The serum pool from patients with ENL reactions showed decreased recognition of M. leprae antigens with IgG 1, IgG2 and IgG3 antibodies compared to nonreactional LL/ BL patients who showed strong recognition with IgGl, IgG2 and IgG3 antibodies. As reported previously (";) the range of antigens recognized by the three IgG subclasses in nonreactional LL/BL patients was different. Although IgG2 antibody also showed lower binding in ENL patients (Fig. 2) the decrease was mostly at the quantitative level and as shown previously, this decrease is related to chemotherapy. Very little binding was observed with IgG4 antibody, even in the LL/BL scrum pool, and no bands were observed in the ENL serum pool.
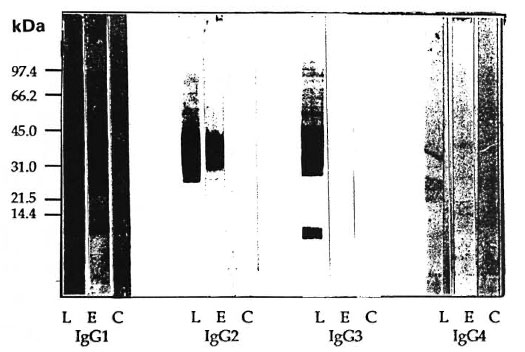
Fig. 2. IgG subclass antibody recognition of M. leprae antigens by serum pools of lepromatous patients withor without ENL reaction and healthy endemic controls. Immunoblot analysis was carried out using mousemonoclonal antibodies specific for human IgG subclass antibodies and enzyme-linked anti-mouse IgG antibodyas a probe. All serum pools were tested at a 1:200 dilution. L = scrum pool of nonreactional lepromatous (LL/BL) patients; E = serum pool of lepromatous patients undergoing ENL reaction; C = serum pool of healthyendemic controls (EC). Numbers on left indicate molecular weights in kilodaltons of known molecular weightmarkers used in the run.
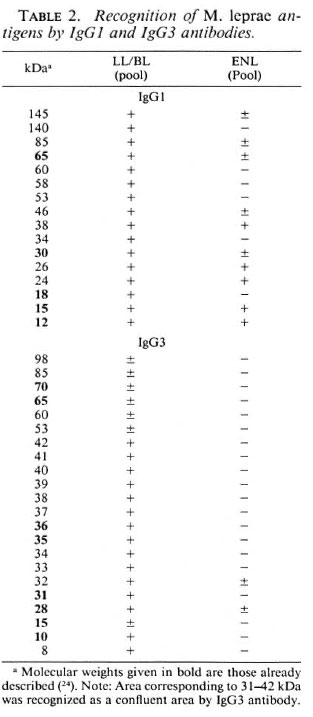
Since decreases in concentrations oflgGl and IgG3 antibody responses are associated with ENL reactions at the quantitative level, we also calculated the molecular weight of the antigens being recognized by these two IgG subclasses in both ENL and nonreactional LL/BL patients (Table 2). At least 40% of the antigens recognized strongly by IgGl antibodies in the nonecactional LL/ BL serum pool were not detected with the ENL serum pool (Table 2). Interestingly, several of the antigens which were recognized by the ENL patients serum pool showed an intensity of binding similar to the LL/BL serum pool. This suggests that there may be a selective decrease of IgG 1 antibodies to certain antigens. A dramatic decrease in the ENL serum pool was observed with IgG3 antibodies. While nonreactional LL/BL patients showed strong binding to proteins at 8, 10, 28, 31, 32 and 40 kDa and weak binding to 15, 53, 60, 65, 70, 85 and 98 kDa proteins, ENL patients showed only weak binding to the 28 and 32 kDa antigens. Very little background binding was observed for IgG3 antibodies in the control serum at the same dilution (1:200).
While serum pools have the advantage of representing the diverse specificities recognized by individual patients within a certain disease group, one disadvantage is that the strong responses may be diluted out by the weak responses. Furthermore, as we have indicated previously, chemotherapy also contributes to a reduction in antibody concentrations and the sera used for making our ENL pools were from patients who had had varying durations of chemotherapy (1-60 months). To overcome both of these limitations, we analyzed five ENL patients individually for IgG3 antibody recognition (Fig. 3). These ENL patients had received no prior chemotherapy for leprosy. Again, very little recognition was observed with the individual ENL patient sera, which is consistent with our results with the serum pools. Only one ENL patient showed strong bands at 32 kDa and 28 kDa, while the remaining patients showed either no binding (#1, #4) or weak binding to 10 kDa (#3) and 32 kDa (#5).
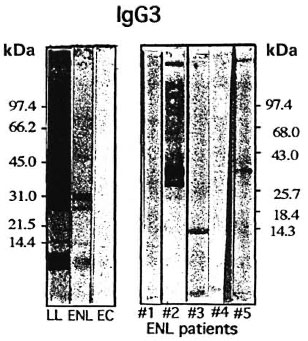
Fig. 3. IgG3 subclass antibody recognition of M.leprae antigens by serum pools of lepromatous patientswith or without ENL reaction, healthy endemic con-trols and five pretreatment lepromatous patients un-dergoing ENL reaction. LL = serum pool of nonreac-tional lepromatous (LL/BL) patients; ENL = serumpool of lepromatous patients undergoing EN L reaction;EC = serum pool of healthy endemic controls; #1, 2,3, 4, and 5 indicate five individual pretreatment ENLpatients' sera. All sera were tested at a 1:200 dilution.Numbers on left and right indicate molecular weightsin kilodaltons of known molecular weight markers usedin the run.
These results demonstrate that both IgG 1 and IgG3 antibodies show decreased recognition of M. leprae antigens at both the quantitative and qualitative levels, which is related to ENL reactions and not to chemotherapy. These antibodies may be important in immune complex formation and deposition in tissues during acute ENL.
DISCUSSION
The most important finding in this study was the selective decrease of IgG 1 and IgG3 subclass antibodies in patients undergoing acute ENL reactions compared to patients with nonreactional lepromatous disease. This decrease was shown to be quantitative by titrating the antibody activity and comparing it against a reference serum with assigned amounts of antibody activity. Such titrations are particularly important when the antibodies show logarithmic distribution, as is the case for IgGl and lgG3 antibodies in leprosy (9). Most of the previous antibody studies were confounded by the variable lengths of chemotherapy undergone by leprosy patients at the time of ENL reaction. Since antibody is known to decline with duration of treatment, a comparable control group is difficult to establish. This is demonstrated when ENL patients were compared with nonreactional LL/BL patients without stratification according to the duration of leprosy chemotherapy. When this was the case, all IgG subclasses showed a significantly lower concentration in ENL patients compared to nonreactional LL/BL patients.
Leprosy patients can also develop ENL prior to chemotherapy (l7). To overcome the effect of treatment on immunoglobulin concentrations, we selected ENL patients who had either received no chemotherapy (N = 13) or <2 weeks of treatment (N = 6) for leprosy. When this group of ENL patients was compared with nonreactional LL/ BL patients with the same treatment status, only IgGl and IgG3 were significantly lowered while the decrease in IgG2 and IgG4 antibodies was related to chemotherapy. This observation was further supported by an immunoblotting analysis using serum pools and individual sera from pretreatment leprosy patients with ENL reaction. While the selective disappearance of binding to certain antigens was observed with IgGl antibodies, most of the binding with IgG3 antibodies to M. leprae antigens disappeared in ENL patients despite strong recognition of several M. leprae antigens in patients with nonreactional lepromatous disease. Both soluble and cell-associated antigens in M. leprae sonicate were recognized by IgG3 antibodies in nonreactional LL/BL patients, which may have implications in terms of soluble immune complex formation and deposition at other sites of the body resulting in the systemic inflammation observed during ENL.
Although there are various reports indicating the decrease of IgG antibodies during ENL (1,10), this is the first study to dissect the response at the IgG subclass level. This is important since IgG is composed of four distinct subclasses which vary in terms of not only their concentrations but also their biological functions (Hamilton, R. G. The human IgG subclasses. Calbiochem Corporation, U.S.A. Doc. no. (1989) CB0051289). IgGl is the dominant immunoglob ulin comprising 70% of the total IgG pool while IgG3 comprises 7%- 10% of the total immunoglobulin pool and, as such, is not a major immunoglobulin in the circulation. However, IgG3 antibody is much more potent than IgGl in its ability to fix complement on a molar basis (2,3). Thus, in diseases in which inflammatory mediators released during complement activation play an important role in tissue damage, the presence of antigen-specific IgGl and IgG3 antibodies at the site of the lesion could be a critical factor.
We have previously shown that in leprosy among the four IgG subclasses, IgGl and IgG3 antibodies were not only selectively elevated but also showed a highly significant correlation with the bacterial load in these patients (9). If B cells are activated at the site of the lesion to secrete M. leprae-specific IgGl and IgG3 antibodies, even low con centrations of IgG3 antibodies would initiate the complement cascade, resulting in the disastrous consequences seen to occur in ENL.
To date very little is known about the factors which result in the precipitation of ENL. Our studies indicate that the factors which initiate the activation of B cells to secrete IgGl and IgG3 antibodies at the site of the lesion may play a crucial role in the precipitation of these events. Several cytokines have been shown to regulate IgG sub class antibodies differentially in humans (21). Future studies in the regulation and switching of IgGl and IgG3 antibody responses may give further insight into the role of these antibodies in the immunopathogenesis of ENL reactions in leprosy.
Acknowledgment. This investigation received financial support from The Rockefeller Foundation and from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). We would like to thank Drs. Thomas J. Chiang, Zeenat Uqaili, Qadeer Ahsan and Kehkashan Awan at Marie Adelaide Leprosy Center, Karachi, Pakistan, for their help in obtaining clinical material. We also want to thank Dr. Sebastian Lucas (UMDS, London, U.K.) for performing histopathology on patients. Excellent technical assistance was provided by Miss Maqboola Dojki and Miss Nabila Abrar in separation and storage of sera and reagents. Secretarial help in manuscript preparation was provided by Miss Regina Paul.
REFERENCES
1. Andreoli, A., Brett, S. J., Draper, P., Paynes, S. N. and Rock, G. A. W. Changes in circulating antibody levels to the major phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int. J. Lepr. 53(1985)211-217.
2. Augener, W., Grey, H. M., Cooper, N. R. and Muller-Eberhard, H. J. The reaction of monomelic and aggregated immunoglobulins with CI. Immunochemistry 8(1971)1011-1020.
3. Bruggemann, M., Williams, G. T., Bindon, C. I., Clark, M. R., Walker, M. R., Jefferis, R., Waldmann, H. and Neuberger, M. S. Comparison of effector functions of human immunoglobulins using a matched set of chimeric antibodies. 166(1987)1351-1361.
4. Chakrabarty, A. K., Marie, M., Saha, K. and Lambert, P. H. Identification of components of IC purified from human sera II. Demonstration of mycobacterial antigens in immune complexes isolated from sera of lepromatous patients. Clin. Exp. Immunol. 51(1983)225-231.
5. Douglas, J. T., Steven, L. M., Fajardo, T., Cellona , R. V., Madarang, M. G., Abalos, R. M. and Steenbergen, G. S. The effect of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59(1988)127-135.
6. Feinstein, A., Richardson, N. and Taussig, M. J. Immunoglobulin flexibility in complement activation. Immunol. Today 7 (1986) 169-171.
7. Huber, H., Douglas, S. D., Nusbacher, J., Kochwa, S. and Rosenfield, R. E. IgG subclass specificity of human monocytes receptor sites. Nature (Lond) 229(1971)419-420.
8. Hussain, R., Dockrell, H. M. and Chiang, T. J. IgG subclass antibody to Mycobacterium leprae 18000 MW antigen is restricted to IgGl and IgG3 in leprosy. Immunology 83(1994)495-500.
9. Hussain, R., Kifayet, A. and Chaing T. J. IgG 1 and IgG3 are the markers of progressive disease in leprosy. Infect. Immun. 63(1995)410-415.
10. Hussain, R., Lucas, S. B., Kifayet, A., Jamil, S., Raynes, J., Uqaili, Z., Dockrell, H. M., Chiang, T. J. and McAdam, K. P. W. J. Clinical and histological discrepancies in diagnosis of ENL reactions clarified by assessment of acute phase proteins SAA and CRP. Int. J. Lepr. 63(1995)222-229.
11. Kifayet, A. and Hussain, R. IgG subclass recognition patterns in leprosy: Recognition of M. leprae antigens by IgGl and IgG3 antibodies is distinct across the disease spectrum. Int. J. Lepr. 64(1996)69-78.
12. Laemmli, U. K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227(1970)680-685.
13. Miller, R. A., Gorder, D. and Harnisch, P. Antibodies to phenolic glycolipid 1 during long term therapy; serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
14. M yrvano, B., G odal, T., R idley, D. S., Froland, S. S. and Song, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14 (1973)541-553.
15. P faltzgraff, R. E. and B ryceson, A. Clinical leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 170-171.
16. R amanathan, V. D., P arkash, O., Ramu, G., P arker, D., C urtis, J., S engupta, U. and T urk, J. L. Isolation and analysis of circulating immune complexes in leprosy. Clin. Immunol. Immunopathol. 32(1984)261-268.
17. Rea, T. H., Levan, N. E. and S chweitzer, R. E. Erythema nodosum leprosum in the absence of chemotherapy: a role of cell mediated immunity. Lancet 2(1972)1252.
18. Ridley, D. S. Skin Biopsy in Leprosy. 3rd edu. Basle, Ciba-Geigy Ltd, 1990.
19. Ridley, D. S. and J opling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
20. Ridley, R. J. and Ridley, D. S. The immunopathology of erythema nodosum leprosum: the role of extra vascular complexes. Lepr. Rev. 54(1983)95-107.
21. Snapper, C. M. and Mond, J.J. Towards a comprehensive view of immunoglobulin class switching. Immunol. Today 14(1993)15-17.
22. Towbin, H., S taehaelia, T. and G ordon, J. Electrophoretic transfer of proteins from Polyacrylamide gels to nitrocellulose sheets; procedure and some applications. Proc. Natl. Acad. Sei. U.S.A. 76(1979)4350-4354.
23. Wemambu, S. N. C, T urk, J. L, W aters, M. F. R. and Rees, R. J. W. Erythema nodusum leprosum: a clinical manifestation of the Arthus phenomenon. Lancet 2 (1969) 933-935.
24. Young, D. B., K aufmann, S. H. E. and Hermans, P. W. M. Mycobacterial protein antigens: a compilation. Mol. Microbiol. 6(1992)133-145.
Ph.D., M.R.C. (Path.), Department of Microbiology, The Aga Khan University, P.O. Box 3500, Karachi 74800, Pakistan.
Reprint requests to Dr. Hussain at above address or FAX 92-21-493-4294.
Received for publication on 21 September 1995.
Accepted for publication in revised form on 9 January 1996.