- Volume 64 , Number 2
- Page: 123–7
Fixed-duration therapy (FDT) in multibacillar y leprosy; efficacy and complications
ABSTRACT
The World Health Organization (WHO) recommended a multidrug therapy (MDT) regimen for multibacillary (MB) leprosy patients in 1982 which was to be administered for a minimum period of 2 years or until a skin smear was negative for acid-fast bacilli, whichever was later. This regimen contains rifampin, dapsone and clofazimine. A single dose of rifampin was shown to effect a high degree of bacterial killing (99.9%). The combined therapy administered for 2 years may be adequate to bring about "total" bacterial killing and to prevent the emergence of drug resistance and persisters. In this study, 360 smear-positive and previously untreated MB leprosy patients were treated with WHO/ MDT for 2 years; 22.8% of these MB patients developed lepra reaction during therapy and 10.7% during surveillance. The bacterial index continued to decline even after termination of fixed-duration therapy. None of these patients relapsed during 886 person-years of surveillance.RÉSUMÉ
L'Organisation Mondiale de la Santé (OMS) a recommandé en 1982 pour la lèpre multibacillaire (MB) une polychimiothérapie (PCT) qui devait être administrée pour une période minimale de 2 ans ou jusqu'à négativation des frottis cutanés, si ceux-ci n'étaient pas négatives après deux ans. Ce régime contient de la rifampicine, de la dapsone et de la clofazimine. On a montré qu'une dose unique de rifampicine avait un pouvoir bactéricide important (99.9%). Le traitement combiné de deux ans peut être adéquat pour parvenir à une destruction bactérienne "totale" et pour prévenir l'apparition de la résistance bactérienne et de germes "persistants." Dans cette étude, on a traité avec une PCT de deux ans 360 malades de la lèpre MB, positifs aux frottis cutanés et non encore traités antérieurement; 22.8% de ces patients MB ont développé une réaction lépreuse durant le traitement, et 9.6% durant la période de surveillance. Aucun de ces patients n'a recidivé au cours de 864 personnes-années de surveillance.RESUMEN
La Organización Mundial de la Salud (OMS) recomendó en 1982 el tratamiento con poliquímioterapia (PQT) de la lepra multibacilar (MB). La PQT debía administrarse durante un periodo mínimo de 2 años o hasta que las extensiones de linfa cutánea se tornaran negativas para bacilos ácido-resistentes (BAAR). La PQT incluye rifampina, dapsona y clofazimina. Ya que se ha demostrado que una sola dosis de rifampina tiene un alto grado de actividad bactericida (99.9%) se considera que la terapia combinada administrada durante 2 años, puede conducir a la muerte de la totalidad de los microorganismos y puede ayudar a prevenir la emergencia de la resistencia a la droga y de los casos persistentes. En este estudio, se trataron 360 pacientes MB, BAAR positivos y sin tratamiento previo, con PQT durante 2 años; el 22.8% de los pacientes desarrollaron algún tipo de reacción leprosa durante el tratamiento y 9.6% durante el seguimiento. Ninguna de estos pacientes mostró evidencias de recaída durante los 864 personas-años de seguimiento.The World Health Organization (WHO) introduced a multidrug therapy (MDT) regimen for multibacillary (MB) leprosy in 1982 containing rifampin, dapsone (DDS) and clofazimine. The recommended duration was for a minimum period of 2 years or until the skin smears were negative for acidfast bacilli (AFB), whichever was later.
One of the disadvantages of DDS monotherapy was the long duration of therapy, i.e., 5 to 10 years or more. The risk of relapse (1,2,9,18) varied from 0.7% per year to 4.9% per year after DDS monotherapy. In addition, a proportion of DDS-resistant relapse(15) might be receiving only DDS monotherapy. The introduction of MDT had remarkably shortened the duration of therapy, but still a considerable proportion of them had to be treated for longer than 2 years(4). The revised recommendations of WHO stated that the proviso to continue MDT beyond 2 years until smear negativity could be dropped.
We present the outcome of fixed duration therapy (FDT) in MB leprosy where the duration of therapy was limited to only 2 years.
MATERIALS AND METHODS
At the Schieffelin Leprosy Research & Training Center (SLR&T), Karigiri, India, all of the newly detected MB leprosy patients have been treated with FDT since 1984, as per the following criteria for the selection of MB cases: a) skin-smear positive (for AFB) lepromatous (LL) and borderline lepromatous (BL) cases; b) previously untreated; c) received DDS monotherapy for <24 weeks; and d) no contrain dications to antilcprosy drugs used in the regimen.
Duration of therapy. WHO-rccommended MDT was administered for 2 years only. The therapy was terminated [patients were released from treatment (RFT)] at the end of 2 years, irrespective of clinical and bacteriological status.
Relapse. Relapse was defined as an increase in the bacterial index (BI; Ridley's scale) of more than 1 + with or without clinical signs.
Surveillance. These patients were clinically reviewed every month (during surveillance) until they became skin-smear negative for AFB and once every third or sixth month subsequently. A skin-smear examination was done once every year.
RESULTS
During the period from 1984 to 1994, 360 MB leprosy patients were included in the study. Among these patients, 18.9% had LL and 81.1% had BL leprosy. The BI was up to 2+ in 59.16% of them, and 40.83% had a BI of >2 + at the start of therapy.
The prescribed treatment was successfully completed by 262 patients, 19.5% LL and 80.5% BL. The BI was up to 2+ in 60.7% of them, and 39.3% had a BI of >2+ at the start of therapy. Some of these patients (27.2%) did not complete treatment for the following reasons: migrated = 6.7%, death = 3.3%, refusal of therapy = 4.7%; 10.6% of the study patients were on treatment and 1.9% had to be removed from the trial since they had to be given an alternate regimen due to drug allergy or lepra reactions. Bacteriological status/clearance. These patients were grouped according to the initial BL The annual skin-smear results (mean BI) of each group are compared with that of similar groups of patients who received MDT until bacteriological negativity (Figs. 1 and 2). The mean BI of the annual skinsmear results showed a decline even after cessation of therapy, and the decline was faster during the initial few years. A majority (71.6%) of the patients with an initial Bl of up to 2.0 + became smear negative within 3 years after starting therapy (FDT); for those with a Bl of >2 + , 26.2% became'smear negative.
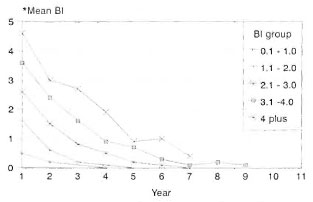
Fig. 1. Bacteriological clearance (fixed durationtherapy in MB leprosy).
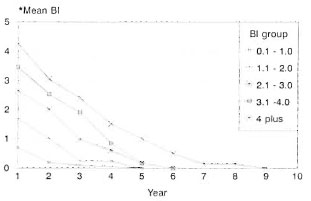
Fig. 2. Bacteriological clearance (MDT until smearnegativity in MB leprosy).
Lepra reaction during therapy. Lepra reactions were experienced by 22.8% of the patients during therapy. Type 1 reactions were observed in 12.3% of the patients, ENL reactions in 3.8% and pure neuritis in 6.7% (The Table). Lepra reactions presenting with only skin manifestations were seen in 8.3% of the patients; 7.8% had both skin and nerve manifestations. Lepra reactions involving nerves with or without skin manifestations were seen in 14.4% of the study patients.
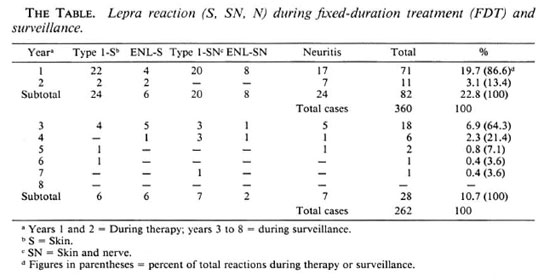
The occurrence of lepra reactions was similar in both BL and LL patients (22.3% BL, 25% LL) during treatment. The occurrence of neuritis in BL patients was 15.3%; in LL patients it was 10.3%. There was no significant difference in the occurrence of lepra reaction among the different Bl groups, e.g., the group with a Bl of >3 + . A majority of the reactive episodes (86.6%) occurred during the first year after starting therapy; 13.4% of lepra reactions occurred during the second year. All of the episodes of lepra reactions were treated with steroids (prednisolone).
Among BL patients who had lepra reactions, 26.2% developed a new disability/deformity (10.8% with motor paralysis and 15.4% with residual motor weakness). The rest of the patients recovered without any new deformity. Only two LL patients developed motor weakness. Overall, 8.5% of lepra reactions resulted in motor paralysis and 14.6% resulted in motor weakness. Among the total study patients, 5.2% developed a new deformity (Fig. 3) as a result of lepra reaction during treatment (5.8% of BL, 2.9% of LL patients).
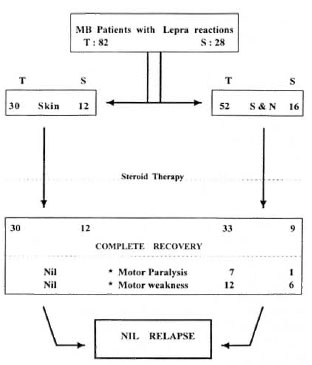
Fig. 3. Outcome of lepra reactions (MB leprosy on FDT). T = During therapy; S = during surveillance; S&N = skin and/or nerve.
Lepra reaction during surveillance. A total of 28 (10.7%) patients developed lepra reactions during surveillance (8.5% BL, 19.6% LL). Type 1 lepra reactions constituted 46.4% of the episodes; 28.6% were ENL reactions and 25.0% were pure neuritis (The Table). Overall, 16 patients had neuritis with or without skin manifestations. Skin smears were positive in 53.6% of them at the time of the reactive episode. A majority (65.5%) of the reactive episodes occurred within the first year of surveillance. All of the episodes of lepra reactions were treated with steroids.
Among the 28 patients with lepra reaction during surveillance, 1 patient (0.4% of total patients completing therapy) had motor paralysis (Fig. 3) and 6 patients (2.3% of total patients completing therapy) were left with residual motor weakness. Among the total study patients who completed therapy, 2.7% developed a new deformity as a result of lepra reaction during surveillance. None of the patients revealed an increase in the Bl either during the lepra reaction or during the subsequent follow up (1 to 8 years).
Observations. 1. The Bl continues to decline after completion of FDT. 2. During treatment 22.8% of MB patients had lepra reaction, 86.6% of which occurred during the first year of therapy. 3. Occurrence of lepra reaction was similar in BL and LL patients and in patients with different Bis. 4. During surveillance, 10.7% of MB patients developed lepra reactions; a majority (64.3%) of the episodes occurred during the first year of surveillance. 5. Among the total lepra reactions, 96.4% (106/110) occurred during the first 4 years after starting combined chemotherapy. 6. New deformity/ disability occurred in 5.2% of our study patients during therapy and 2.7% during surveillance as a result of lepra reaction. None of those patients with motor weakness had visible deformity.
DISCUSSION
Bacteriostatic antileprosy drugs arc very slow in action, and thus the release of bacterial debris/dead bacilli is also presumably less and continues for a long duration. MB leprosy patients generally have a very minimal capability to mount an immune response to Mycobacterium leprae. Hence, even if bacterial killing is brought about by effective chemotherapy, clearance of the bacterial debris/dead bacilli may take place in a slow phase(5).
The duration of therapy in MB leprosy is generally based on the attainment of smear negativity, in other words "complete" elimination of bacilli from the infected person (< 10,000 bacilli per ml of infected tissue). It has been shown that a single dose of rifampin can bring about a bacterial killing to the extent of 99.9%. Chemothcrapeutic regimens of various durations have been studied. Relapse rates after combined chemotherapy regimens administered for more than 18 months have been shown to be less(6) when compared to a shorter duration of MDT. The relapse rate after WHO-recommended MDT(4,7 ,8,17)was reported to vary from <1 % per year to 17.7%. The wide variation in the relapse rates is mainly due to the different criteria employed for diagnosing relapse.
In this study, the WHO-recommended MB regimen was administered to MB leprosy patients for 2 years only. All of these patients continue to show a decline in their Bis even after cessation of therapy and no case of relapse has been encountered.
The WHO-recommcnded MDT regimen containing rifampin plus DDS and clofazimine given for 2 years is expected to achieve 100% bacterial killing. Use of any potent antimycobacterial agent may bring about a lepra reaction, probably due to the effective bacterial killing and the release of a larger load of bacterial debris/dead bacilli. It has been reported that 30% to 53% of LL patients seemed to have developed lepra reactions(13,18) during DDS monotherapy and 10.4% and 41% in MDT(3,12) groups. In this study, 30.5% of the MB leprosy patients who received FDT developed lepra reaction (during therapy and surveillance).
Due to the slow clearance of bacterial debris/dead bacilli and the short duration of chemotherapy, the possibility of late lepra reaction(11,16) still exists even after completion of chemotherapy (FDT). Even though a relapse can manifest as a reactive episode, it need not always be an indicator of relapse. A therapeutic trial with steroids(10) and close observation is necessary to rule out or identify relapse at an early stage.
Conclusion. 1. Bacterial clearance, although slow, continues to occur even after cessation of therapy (FDT). 2. None of the MB patients revealed evidence of relapse during 886 person-years of surveillance. 3. MB patients need to be monitored for the occurrence of lepra reaction for a minimum period of 5 years after commencement of FDT with a combined drug regimen. The findings of this study indicate that 2 years of treatment with the WHO-recommended regimen may be adequate to effect bacterial killing.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). We extend our sincere thanks to the World Health Organization for the financial support provided for this project. We also thank Mr. P. Samuel (Project Coordinator), Mr. Raja Samuel (Assistant Statistician), Mr. Lewis Kumar (Office Secretary) and all of our staff who contributed their effort and skills for successful implementation of the project.
REFERENCES
1. Almeida, J. G., Christian, M. and Chacko, C. J. G. Follow-up of lepromatous (LL and BL) patients on dapsone (DDS) monotherapy after attainment of smear negativity in Gudiyatham Taluk, South India. Int. J. Lepr. 51(1983)382-384.
2. Almeida, J. G., Jesudasan, K., Christian, M. and Chacko, C. J. G. Relapse rates in lepromatous leprosy according to treatment regularity. Int. J. Lepr. 54(1986)16-20.
3. Becx-Bleumink, M. and Berhe, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
4. Chopra, N. K., Agarwal, J. S. and Pandya, P. G. Impact of multidrug therapy on leprosy in Baroda district (Gujarat). Indian J. Lepr. 61(1989)179-189.
5. Ganapathi, R., Pai, R., Gandewar, K. L. and Thressia, X. J. For how long should a multibacillary leprosy patient be treated? Indian J. Lepr. 61(1989)467-471.
6. Katoch, K., Natarajan, M., Bagga, A. and Katoch, V. M. Clinical and bacteriological progress of highly bacillated BL-LL patients discontinuing treatment after different periods of MDT. Int. J. Lepr. 59(1991)248-254.
7. Leprosy Unit, WHO, Division of Control of Tropical Diseases. Risk of relapse in leprosy. Geneva: World Health Organization, 1994. WHO/ CTD/94.1.
8. Marchoux Chemotherapy Study Group. Relapses in multibacillary leprosy patients after stopping treatment with rifampin-containing combined regimens. Int. J. Lepr. 60(1992)525-535.
9. Noordeen, S. K. Relapse in lepromatous leprosy. Lepr. Rev. 42(1971)43-48.
10. Pannikar, V., Jesudasan, K., Vijayakumaran, P. and Christian, M. Relapse or late reversal reaction? Int. J. Lepr. 57(1989)526-528.
11. Pattyn, S. R., Eyckmans, L. and Gigase, P. Late reversal reaction after combined treatment of a patient with multibacillary leprosy. Ann. Soc. Belg. Med. Trop. 64(1984)291-294.
12. Ramesh, V., Misra, R. S. and Saxena, U. Multidrug therapy in multibacillary leprosy; experience in an urban leprosy center. Int. J. Lepr. 60(1992)13-17.
13. Susman, I. A. Clinical observations on erythema nodosum leprosum (ENL). Lepr. Rev. 29 (1958) 227-231.
14. va n Brakel, W., Kist, P., Noble, S. and O'Toole L. Relapses after multidrug therapy for leprosy: a preliminary report of 22 cases in west Nepal. Lepr. Rev. 60(1989)45-50.
15. va n Diepen, T. W. and Mengistu, G. Relapse rate and incidence of dapsone resistance in lepromatous leprosy patients in Addis Ababa: risk factors and effect of short-term supplementary treatment. Int. J. Lepr. 53(1985)189-197.
16. Vijayakumaran, P., Manimozhi, N. and Jesudasan, K. Incidence of late lepra reaction among multibacillary leprosy patients after MDT. Int. J. Lepr. 63(1995)18-22.
17. Waters, M. F. R. Relapse following various types of multidrug therapy in multibacillary leprosy. (Editorial) Lepr. Rev. 66(1995)1-9.
18. Waters, M. F. R., Rees, R. J. W., Liang, A. B. G., Khoo, K. F., Meade, T. W., Parikshak, N. and North, W. R. S. The rate or relapse in lepromatous leprosy following completion of twenty years of supervised sulphone therapy. Lepr. Rev. 57(1986)101-109.
19. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
20. WHO Study Group. Chemotherapy of leprosy. Geneva: World Health Organization, 1994, pp. 5-7. Tech. Rep. Ser. 847.
1. M.B.B.S., D.P.H., Associate Epidemiologist.
2. M.B.B.S., D.H.E., Associate Epidemiologist.
3. M.B.B.S., D.T.P.H., Ph.D. (London), Head, Branch of Epidemiology and Leprosy Control, Schieffelin Leprosy Research & Training Center, Karigiri 632106, South India.
Received for publication on 14 June 1995.
Accepted for publication on 13 December 1995.