- Volume 64 , Number 2
- Page: 128–32
Effectiveness of MDT in multibacillary leprosy
ABSTRACT
The study on the use of World Health Organization multidrug therapy (WHO/ MDT) under field conditions was initiated in December 1981, and included 1067 multibacillary (MB) patients treated with two MDT regimens. The first was a THELEPrecommended regimen which consisted of 600 mg of rifampin (RFP) and 600 mg of clofazimine (CLO) given under supervision on two consecutive days monthly and 225 mg of diacetyl diaminodiphenylsulfone (DADDS) bimonthly plus dapsone (DDS) 100 mg daily unsupervised. The second regimen was the conventional MDT: patients received RFP 600 mg and CLO 300 mg supervised once a month, daily 100 mg of DDS and 50 mg of CLO unsupervised.A zero relapse rate was obtained after more than 10 years (a total of 8244 personyears) of follow up. Both regimens were well tolerated with few complications and a high acceptability, even among women. The fall in the bacterial index (BI) was 0.5-1.0+ in positive patients. CLO discoloration began to decrease after 3 months and disappeared within 1 year after it was discontinued. Seventy-two patients (67%) developed reactions during the treatment period; a further 12 patients developed post-treatment reactions during the surveillance period. This study vindicates MDT treatment for MB patients as recommended by WHO under field conditions.
RÉSUMÉ
L'étude de l'utilisation de la polychimiothérapic de l'Organisation Mondiale de la Santé (PCT/OMS) dans les conditions de terrain a commencé en décembre 1981, et comportait 1067 patients multibacillaircs (MB) traités par deux régimes PCT. Le premier était un régime recommandé par THELEP et consistait en 600 mg de rifampicine (RFP) et 600 mg de clofazimine (CLO) donnés chaque mois sous supervision deux jours consécutivement et 225 mg de diacétyl-diamino-diphénylsulfone (DADDS) deux fois par mois et 100 mg de dapsone (DDS) par jour de manière non supervisée. Le second régime était la PCT conventionnelle: les patients recevaient 600 mg de RFP et 300 mg de CLO de manière supervisée une fois par mois, et 100 mg de dapsone et 50 mg de CLO chaque jour sans supervision.Un taux nul de récidive a été obtenu après plus de 10 ans (un total de 8244 personnes-années) de suivi. Les deux régimes furent bien tolérés, avec peu de complications et une acceptabilité élevée, même parmi les femmes. La chute de l'indice bactérien (1B) fut de 0.51.0+ chez les patients positifs. La coloration duc à la CLO commença à diminuer après trois mois et avait disparu un an après son arrêt. Septante-deux patients (6.7%) ont développé des réactions durant le traitement; douze autres patients ont développé des réactions durant la période de surveillance. Cette étude soutient le traitement PCT tel que recommandé par l'OMS pour les patients MB dans les conditions de terrain.
RESUMEN
En diciembre de 1981 se inició un estudio de campo sobre el use de dos esquemas de tratamiento con poliquimioterapia (PQT) que incluyó 1067 pacientes multibacilares(MB). El primer esquema, recomendado por THELEP, consistió en la administración supervisada de 600 mg de rifampina (RFP) y 600 mg de clofazimina (CLO) durante 2 días consecutivos de cada mes y 225 mg de diaectil diaminodifenilsulfona (DADDS) cada dos meses, más 100 mg diarios de dapsona (DDS). El segundo esquema fue la PQT convencional de la OMS: 600 mg de RFP y 300 mg de CLO una vez al mes (bajo supervisión) y 100 mg de DDS y 50 mg CLO diarios, sin supervisión.Después de más de 10 años de seguimiento (un total de 8244 personas-años) se obtuvo una tasa de recaída de cero. Ambos esquemas de tratamiento fueron bien tolerados, con pocas complicaciones, y bien aceptados aún entre las mujeres. La caída en el índice bacteriológico (BI) fue de 0.5-1.0+ en los pacientes BAAR positivios. La decoloración debida a la CLO comenzó a disminuir después de 3 meses de tratamiento y desapareció en el transcurso de un año. Sesenta y dos pacientes (67%) desarrollaron reacciones durante el periodo de tratamiento; 12 pacientes más desarrollaron reacciones post-tratamient durante el periodo de seguimiento. Este estudio reivindica la PQT recomendad por la OMS para el tratamiento de los pacientes MB.
Multidrug therapy (MDT) was first recommended by the World Health Organization (WHO) Study Group in 1981 (14). Since 1985 some 5.6 million cases have been cured with MDT (l2). In 1994, approximately 54% of the 1.69 million registered leprosy patients are on MDT (12). The MDT regimens have proven to be extremely effective throughout the world.
The Schieffelin Leprosy Research & Training Centre, Karigiri, India, has been doing leprosy control in the area since 1962. Until the early 1980, when MDT was introduced, both multibacillary (MB) and paucibacillary (PB) leprosy patients were treated with dapsone monotherapy. PB patients were released from treatment after varying periods of dapsone monotherapy (5) and MB patients were treated for life (1).
The field trials of combined chemotherapy (MDT) in MB leprosy patients funded by the WHO began in December 1981 at Karigiri. The intake was completed in December 1982. A total of 1067 borderline lepromatous (BL) and lepromatous (LL) patients were included in the study.
This study was done with careful planning and supervision under field conditions of a leprosy control program. All of the MB patients on treatment in the control program were included in the study which included patients who were skin smear negative after treatment with dapsone monotherapy. The 1067 MB patients were put on two different MB regimens: 562 (53%) of them were put on Regimen A and 505 (47%) patients on Regimen B (see Materials and Methods); 357 (33.5%) of the patients were smear positive at initiation of treatment. During the treatment period, patients were seen monthly, and the clinical status and complications were recorded. Patients were treated for a minimum of 2 years or until skin smears became negative. After release from treatment, patients were seen once every 3 months for a period of 5 years after which they were seen annually. A maximum of 12 years of follow up was available after release from treatment.
MATERIALS AND METHODS
The leprosy control program in Gudiyatham Thaluk has been described previously(5,10). This study was part of a WHO-sponsored trial in MB leprosy patients using MDT which was initiated in 1981. All MB patients in the leprosy control area of Gudiyatham Thaluk were screened before starting MDT, and patients with medical contraindications to MDT, patients older than 60 years of age and women pregnant at the time of screening were excluded. Two regimens were used to treat the 1067 MB patients. The first regimen (Regimen A) was a monthly supervised dosage of 600 mg rifampin and 600 mg of clofazimine given on two consecutive days (both doses were supervised), bimonthly injections of 225 mg of DADDS, and a daily dose of 100 mg of dapsone unsupervised. This regimen was used on the recommendation of the WHO as a highly supervised regimen. The daily dapsone was the only nonsupervised drug. The second regimen (Regimen B) was the standard WHO regimen (11,14,15) containing a monthly supervised pulse of 300 mg of clofazimine and 600 mg rifampin, along with a daily unsupervised dosage of 50 mg clofazimine and 100 mg dapsone. Patients were seen regularly during treatment and follow up which was done once every month when the patients were seen by a trained doctor. A detailed clinical assessment and a skin smear were done every year. This procedure was carried out while the patient was on treatment and continues to the present. The intake of dapsone was monitored regularly using the tablet count and the urinary dapsone/creatinine ratio.
Allocation of the patients into Regimen A or Regimen B was based on geographic location. The leprosy control area of Gudiyatham Thaluk is divided into four blocks. Patients from block 1 and block 2 were put on Regimen A and patients from blocks 3 and 4 were put on Regimen B. Of the original 1067 patients, only 44 (4.1%) had not received previous monotherapy with dapsone.
Statistical tests were done using the program EPI INFO version 6. The chi-squared test for 2 x 2 tables were used.
RESULTS
Of the 1067 patients, 772 (72%) were males and 295 (28%) were females; 775 of the patients were classified as lepromatous leprosy (LL, 73%) and 292 were classified as borderline lepromatous leprosy (BL, 27%). The distribution of patients by initial bacterial index (BI) prior to commencing MDT is given in Table 1; 710 (67%) were skin-smear negative since they had received prior treatment with dapsone monotherapy, 116 patients (11%) had a BI of over 2 + .
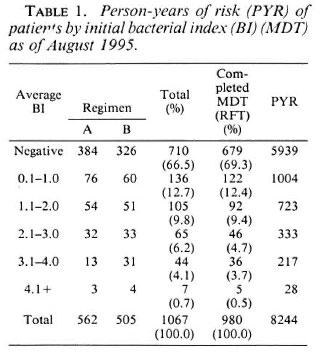
During treatment 72 patients developed reactions (6.7% of the 1067 patients); 36 (50%) of them had erythema nodosum leprosum (ENL) reactions, 20 (27.8%) had reversal reactions (RR), and 16 patients had neuritis. There was no statistically significant difference in reactions in patients on Regimens A or B. Table 2 shows the episodes of RR, ENL, and neuritis and the period after initiation of treatment in which they occurred; 15.3% had reactions at the start of treatment and an additional 58.3% during the first year of treatment, 8.3% of reactions occurred after the second year of treatment. Twelve patients developed posttreatment reactions (PTR) during the period of surveillance (follow up after release from treatment); 7 out of the 12 reactions occurred during the first 3 years of follow up. Of the 12 patients who developed PTR, 3 of the reactions were localized to the skin, 2 patients had reactions in the skin and nerves, and 7 patients had only neuritis. All of these patients were treated with steroids for 4-16 weeks, during which period the reactions subsided. Six of the patients who developed PTR were BL; the remaining six were classified as LL.
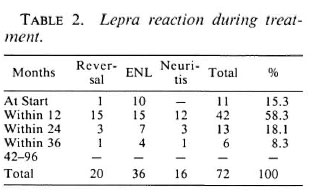
The bacterial clearance of the patients is shown in Table 3. There was no difference in the clearance in Regimen A or Regimen B patients. The bacterial clearance ranged from an average BI of 0.5 to 1+ fall per year. BL patients had a more rapid clearance than MB patients. However, the average BI of BL patients was lower than in LL patients.
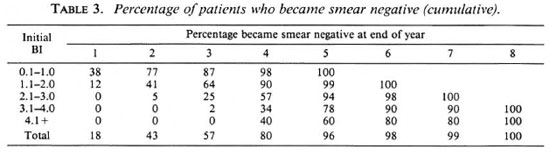
Of the 1067 MB patients put on MDT 980 (91.8%) completed their full course of treatment (Table 1). As of 31 December 1994, 723 of the 980 patients who completed treatment (73.8%) were still on surveillance. At the end of July 1995, the patients had been followed up for a period of 8244 person-years after release from treatment. Patients who were smear negative to start with had a greater duration of follow up. Patients with an average BI of 2.1 and above contributed 578 person-years of follow up. No relapses have been identified so far. For the purposes of this study, relapse was defined as an increase in the BI of 1 + or more at any site seen on two consecutive skin-smear examinations at a 6-month interval, with or without clinical evidence of reactivation.
The average clinic attendance during the period of treatment and follow up ranged between 83% to 99.8%. It was higher during the treatment period.
Clofazimine discoloration was dependent upon the activity of the disease and was more intense in active patients. After cessation of treatment, clofazimine discoloration began to decrease after 3 months and disappeared within a year. The disappearance of clofazimine discoloration was often followed by the reappearance of old inactive lesions, especially in BL patients. Dryness and icthyosis of the extremities was common. Abdominal discomfort and mild diarrhea episodes were frequent at the commencement of MDT. There were 23 children born to 17 mothers while the mothers were on MDT. All of the children showed clofazimine discoloration which persisted as long as the mother remained on MDT and the children received breast milk. The breast milk was also slightly pink due to the excretion of clofazimine.
During treatment, 14 patients developed jaundice; however, this could not be attributed to MDT. One patient was diagnosed as hypersensitive to dapsone and the therapy was continued with clofazimine and rif-
During treatment, 87 patients were deleted from the study. Of them, 37 (42.5%) had migrated, 28 (32.2%) had died, and 22 (25.3%) had refused to continue treatment. The ages of the 87 patients deleted during treatment were statistically significantly lower than those who were deleted after completion of treatment (p <0.05, chisquared test). There were no significant differences in sex, type, or regimen. The reason for refusal was: discoloration, 3 patients; problems with swallowing the medicines, 1 patient; pain of DADDS injection, 2 patients; eye problems, 1 patient (patient attributed his eye problems to the drugs); nausea, 1 patient; and nonspecific reasons, 14 patients (63.6%). Among the 22 patients who refused treatment, 10 were from Regimen A and 12 were from Regimen B. MDT was re-started in five patients after motivation. Among the patients who died, over 70% were over the age of 50 years.
DISCUSSION
Response to treatment as measured by the fall in BI was similar in both Regimen A and Regimen B. Regimen A has only dapsone as the unsupervised component, and it was thought that responses would be better than when both clofazimine and dapsone were given unsupervised. Of the original cohort of 1067 MB patients, 710 (67%) were skin-smear negative at the start of the trial and only 44 patients had not been treated with dapsone earlier. The fall in the BI was similar to the fall in the BI of patients treated with dapsone monotherapy (7).
The occurrence of complications related to MDT were minimal and drug reactions were uncommon. MDT was well tolerated and accepted, even among women. Clofazimine discoloration did not pose a problem, but the women were educated in respect to the discoloration so as to avoid problems at home. This was especially so in those women who became pregnant during therapy.
Reactions developed in 6.7% of the patients. This was much lower than the patients on dapsone monotherapy (10), probably due to the antiinflammatory action of clofazimine.
In the 14 patients who developed jaundice, treatment was suspended until liver function tests returned to normal at which time MDT was re-started. None of the patients had a recurrence of jaundice, thus the jaundice was probably due to hepatitis A.
Results from a large number of patients treated in the field with MDT are available, giving a low relapse rate (3,6)- The review paper by WHO (13) gave a relapse rate (RR) of 2.3/1000 person-years of risk (PYR) after nearly 200,000 PYR of follow up in MB patients put on MDT. Earlier studies (2), in the same leprosy control area of Karigiri, of relapses in MB patients while on dapsone monotherapy for life, gave the prevalence rate of 2.3% among 1431 MB patients studied. In that study (2), relapse was defined as "patients presenting with reactivation, with an increase of BI of two or more, while on treatment, after 260 weeks of dapsone monotherapy."
This study, done with careful supervision under field conditions, shows the efficacy of MDT. The zero relapse rate is very low for use of any drug for any disease. The findings of the Marchoux Chemotherapy Study Group (4) indicated that relapse following MDT correlated with a high BI. A separate study on 34 MB patients with a BI of over 3 + , treated with MDT for 2 years (submitted for publication) also has shown no relapse. However, the follow up was only for 4 years and, thus, a longer follow up is needed.
In the current study the follow up was as long as 12 years. It remains to be seen whether late relapses will occur and, hence, these patients are still being followed up.
Acknowledgment. We remember with gratitude the contribution of the late Dr. M. Christian in these and other studies done at Karigiri. We wish to thank Dr. V. K. Pannikar who was involved in the study from its inception until 1991. We also want to thank all of the medical officers, nonmedical supervisors, paramedical workers, and office staff who contributed to the implementation of the project; Mr. Raja Samuel Bushanam for the statistical input and Mr. C. Lewis Kumar for the secretarial help. This trial received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
REFERENCES
1. Almeida,J. G., Jesudasan, K., Christian, M. and Chacko, C. J. G. Relapse rate in Iepromatous leprosy. Int. J. Lepr. 54(1986)16-20.
2. Balraj, V., Jesudasan, K., Chacko, C. J. G., Christian, M., Taylor, P. M., Fritschi, E. P. and Job, C. K. Prevalence of secondary dapsone resistance in Gudiyatham Taluk; preliminary report. Int. J. Lepr. 48(1980)397-401.
3. Becx-Bleumink, M. Relapses among leprosy patients treated with MDT: experiences in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Centre in Ethiopia. Int. J. Lepr. 60(1992)421-435.
4. Jamet, P., Ji, B. and the Marchoux Chemotherapy Study Group. Relapse after long-term follow up of multibacillary leprosy patients treated by WHO multidrug regimen. Int. J. Lepr. 63(1995)195-201.
5. Jesudasan, K, Bradley, D. J. and Christian, M. Relapse rates among nonlepromatous leprosy patients released from control. Int. J. Lepr. 52(1984)304-310.
6. Karat, A. B. A., Rao, P. S. S., Karat, S. and Job, C. K. Epidemiological studies in Gudiyatham Thaluk; pattern of familial aggregation. Part 2. Lepr. Rev. 40(1969)93-98.
7. Kurz, X. M., deClercq, E. E. and Vellut, C. M. Rate and time distribution of relapses in multibacillary leprosy. Int. J. Lepr. 57 1989)599-606.
8. Pfaltzgraff, R. E. and Bryceson, A. Clinical leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 165-172.
9. Rao, P. S. S., Karat, A. B. A., Karat, S. and Job, C. K. Epidemiological studies in Gudiyatham Thaluk; pattern of familial aggregation. Part1. Lepr. Rev. 38(1967)77-82.
10. Rees, R. J. W. The microbiology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, p. 46.
11. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
12. WHO Leprosy Unit. Global strategy for the elimination of leprosy as a public health problem. Geneva: World Health Organization, 1994. WHO/ CTD/LEP/94.2.
13. WHO Leprosy Unit. Risk of relapse in leprosy. Geneva: World Health Organization, 1994. WHO/ CTD/LEP/94.1.
14. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
15. World Health Organization. Chemotherapy of leprosy. Geneva: World Health Organization, 1994. Tech. Rep. Ser. 847.
1. M.B.B.S., D.T.P.H., Ph.D.;Schieffelin Leprosy Research and Training Centre, Karigiri, N. A. A. District, Tamil Nadu 632106, India.
2. M.B.B.S., D.P.H.; Schieffelin Leprosy Research and Training Centre, Karigiri, N. A. A. District, Tamil Nadu 632106, India.
3. M.B.B.S., D.H.E.; Schieffelin Leprosy Research and Training Centre, Karigiri, N. A. A. District, Tamil Nadu 632106, India.
4. M.A., M.P.H., Dr.Ph., F.S.S., F.S.M.S.; Schieffelin Leprosy Research and Training Centre, Karigiri, N. A. A. District, Tamil Nadu 632106, India.
5. Schieffelin Leprosy Research and Training Centre, Karigiri, N. A. A. District, Tamil Nadu 632106, India.
Received for publication on 29 March 1995.
Accepted for publication in revised form on 5 December 1995.