- Volume 64 , Number 2
- Page: 136–41
Drug resistance in nepali leprosy patients
ABSTRACT
Although multidrug therapy (MDT) was introduced into Nepal in 1983, the MDT coverage only recently exceeded 67%. In view of the large number of patients who were still receiving dapsone monotherapy, it is relevant to investigate the current levels of dapsone and rifampin resistance. The study was undertaken at a leprosy referral hospital near Kathmandu. Over a 5 ½ -year period, 157 leprosy patients with a bacterial index (BI) >2.0 were investigated for drug resistance according to the method of Rees. Among previously untreated cases, 6% of 88 isolates showed low-dose dapsone resistance; among previously treated patients with a presumed relapse, 47% of 34 isolates demonstrated dapsone resistance. In the remaining 35 cases there was no growth in control mice. Rifampin resistance was not confirmed in any case.RÉSUMÉ
Bien que la polychimiothérapic (PCT) ait été introduite au Népal en 1983, la couverture PCT n'a que récemment dépassé 67%. Au vu du grand nombre de patients qui recevaient encore une monothérapie à la dapsone, il est approprié d'évaluer les taux actuels de résistance à la dapsone et à la rifampicine. L'étude a été entreprise dans un hôpital de référence pour la lèpre près de Katmandu. Sur une période de 5 ½ ans, 157 malades de la lèpre avec un indice bactérien (IB) > 2.0 ont été examinés selon la méthode de Rees pour Inexistence d'une résistance aux médicaments. Parmi les cas non traités auparavant, 6% des 88 isolats ont montré une faible résistance à la dapsone; parmi les patients traités antérieurement suspectés de récidive, 47% des 34 isolats ont montré une résistance à la dapsone. Pour les 35 cas restants, il n'y eut aucune croissance chez les souris témoins. La résistance à la rifampicine n'a été confirmée dans aucun cas.RESUMEN
Aunque la poliquimiotcrapia (PQT) se introdujo cn Nepal en 1983, solo recientemente la covertura de la PQT excedió el 67%. En vista del gran número de pacientes que todavia estaban recibiendo monoterapia con dapsona, era importante investigar las tasas actuates de resistência a la dapsona y a la rifampina. El estúdio se llevó a cabo en un hospital de referencia cerca de Kathmandu. En un periodo de 5 anos y medio se investigo la resistência a drogas (por el método de Rees) en 157 pacientes con índices bacteriológicos iguales o mayores de 2.0. Entre los casos sin tratamiento prévio se encontro que cl 6% de 88 aislados mostraron resistência a bajas dosis de dapsona; entre los casos pretratados en recaida, cl 47% de 34 aislados mostraron resistência a la dapsona; en los 35 casos restantes no se demostro resistência a la dapsona. En ningún caso se observo resistência a la rifampina.Dapsone resistance has been recognized as a problem in leprosy programs since the first proven case reported from Malaysia in 1964 (9). Prevalence rates of dapsone resistance vary widely (l6) from place to place, and may be affected by features of the leprosy control program in the area. Early reports (1,2,6,7) from Africa and India gave levels of l%-7% among treated lepromatous cases. Over a 9-year period, the prevalence of secondary dapsone resistance apparently increased from 0.1 %-10% in a Malaysian program. (58,10)
In Nepal, dapsone was introduced around 1957 and multidrug therapy (MDT [as recommended by the World Health Organization (WHO)] has been made available in some areas since 1983. However, due to logistic difficulties and the lack of adequately trained staff, MDT coverage only recently exceeded 67% (data supplied by Leprosy Section, Ministry of Health, HMG/Nepal). Hence, there may be many leprosy patients in Nepal with primary or secondary dapsone resistance.
The chance of rifampin resistance developing during MDT for leprosy is probably small. However there is a risk of undiagnosed leprosy patients, who also have tuberculosis, receiving rifampin without other anti leprosy drugs.
Little information was available on the development and spread of drug resistance in Nepal. Hence this work was undertaken in 1987-1993 to assess the size of the current problem and to predict future trends.
MATERIALS AND METHODS
Patients. Recently diagnosed, previously untreated, multibacillary (MB) leprosy patients from anywhere in Nepal who attended a leprosy referral clinic in the Kathmandu Valley were eligible. In addition, previously treated leprosy patients with prima facie evidence of MB relapse or reactivation were eligible. In both groups, the patient's verbal informed consent was required. Patients were clinically classified according to Ridley-Jopling classification (12) and histological confirmation was sought.
Biopsy method. A small piece of skin was taken from any convenient site where the bacterial index (BI) was >2 + and put into a dry sterile container. The biopsies were processed the same day or (if necessary) stored overnight at 4ºC. Individual bacillary counts and mouse foot pad (MFP) inoculations were performed according to the method of Rees. After homogenization in 2 ml of 0.1% sterile phosphate buffered solution (PBS) or normal saline at pH 7.2, a sample was stained by the Ziehl-Neelsen method for counting of acid-fast bacilli (AFB). A suspension of 104 AFB in 30 fil of 0.1% PBS or normal saline was injected into each hind foot pad of Swiss albino mice. Samples of the AFB extracted from each patient's biopsy also were inoculated onto Lowenstein-Jensen medium to exclude contamination by Mycobacterium tuberculosis.
Each sample of M. leprae was inoculated into five groups of mice: 1 group of 10 on normal feed served as control group, 3 groups receiving different concentrations of dapsone and 1 receiving one concentration of rifampin, each with 5-6 mice.
Dapsone (The Welcome Foundation Ltd, London, U.K.) was administered continuously in the diet at concentrations of 0.0001%, 0.001% and 0.01%. Rifampin (provided by the National Institute for Medical Research, London, and Sigma Chemicals, R-3501, Lot 80H 3294) was freshly prepared by grinding in water and administered weekly by gavage with an esophageal cannula. In early experiments, the individual doses were 0.3 ml of a 0.05% solution, appropriate to an average body weight of 25 g. Later, the weekly dose was increased to 0.4 mg in 0.4 ml (10 mg kg/ body weight) when we found that the average mouse weight was 37 g.
Harvests were initiated in the control group after 6 months and were continued at monthly intervals until a growth of 1.5 logs in the number of AFB was detected. At this point all of the mice were sacrificed and their foot pads were harvested (10 foot pads per group). The foot pads were pooled for each mouse, although this may disguise a small growth in one foot pad.
If the M. leprae isolate proved sensitive to 0.0001% dapsone, the remaining dapsone groups were not harvested. The result was considered negative if the bacterial count reached <1 x 105AFB/foot pad in individual mice. Growth to at least 105 in three or more mice was considered positive.
RESULTS
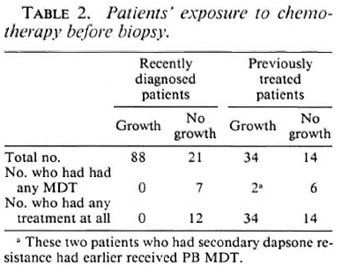
In 35 cases (22%) no significant growth was obtained in control mice and, therefore, no conclusions could be drawn about drug resistance of the inoculum of patients involved in these experiments (Table 1). Among these, 12 of 21 recently diagnosed cases had had 1-8 weeks of MB MDT, and 4 of 14 previously treated active cases had been receiving treatment at the time the biopsy was taken, and 2 of 14 had had some MDT at an earlier date (Table 2). This may explain the failure of growth of M. leprae in control mice.
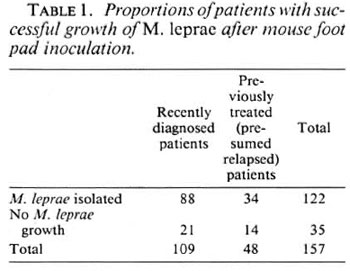
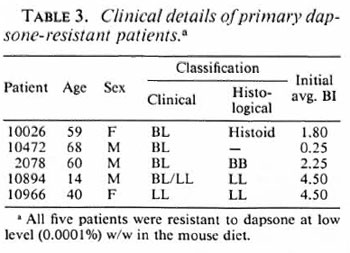
Dapsone resistance in recently diagnosed cases. In 5 (6%) of the 88 fresh cases from which M. leprae were isolated, there was significant growth of M. leprae in dapsonefed mice, but the resistance was only at a low level (0.0001%). All of these patients denied receiving any previous treatment for leprosy. Three of them had no known contact with another leprosy-affected person. The five patients did not live in the same area. The clinical details of these five patients are given in Table 3.
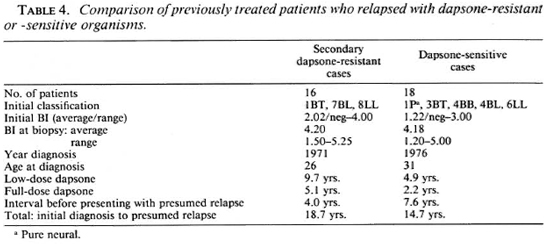
Dapsone resistance in previously treated cases. Out of the 34 isolates obtained from previously treated patients, 16 (47%) showed growth of M. leprae in dapsone-fed mice (Table 4). Full details of the patients' earlier treatments were available in all but one case. All had received, by current criteria, inadequate dosages or durations of dapsone treatment but were clinically inactive at the time they discontinued treatment. They had been without treatment for an average of 4 years, and most of them had reported voluntarily for examination, when the MFP biopsy was taken, because of new symptoms such as diffuse infiltration or nodules. One patient (1759) had no new signs or symptoms except for a positive smear.
Of the isolates from relapse cases, eight (23.5%) were resistant to 0.01% dapsone in the mouse diet equivalent to 100 mg of dapsone daily for an average adult patient. Five isolates were resistant to 0.001% dapsone in diet and three isolates were resistant only to 0.0001% dapsone in diet.
Among the patients who relapsed with secondary dapsone resistance, all but one were initially classified as LL or BL leprosy and, in most cases, the clinical classification was confirmed by histological examination. One initially smear-negative, clinically BT, patient (2411) developed a positive smear within 6 years of starting dapsone monotherapy and, after 21 years of irregular dapsone treatment, presented with new nodules and a smear of 4.7+ (clinically BL). Another patient (1813), who was initially a typical LL case with a negative lepromin test, relapsed with borderline-type lesions (histologically BL) and a low positive lepromin test (3x 3 mm induration). He had developed low-level, secondary dapsone resistance during 19 years of dapsone monotherapy and yet had experienced immunological upgrading.
Compared with the dapsone-resistant group, patients whose isolates were dapsone sensitive were older at first diagnosis, had a shorter duration of previous treatment and a lower initial BI, and had a longer interval before presentation with relapse. Age and BI at time of MFP biopsy were similar.
Suspected rifampin resistance. Out of 122 biopsies tested, seven initially showed growth of M. leprae in the presence of rifampin. The mice used in these experiments had received the lower dose of rifampin. On passage into other mice, none of these suspected rifampin-resistant isolates grew in the presence of the higher dose of rifampin (0.4 mg/wk). Hence, rifampin resistance was not confirmed.
DISCUSSION
The presence of dapsone resistance, both primary and secondary, has been demonstrated in the Nepali population.
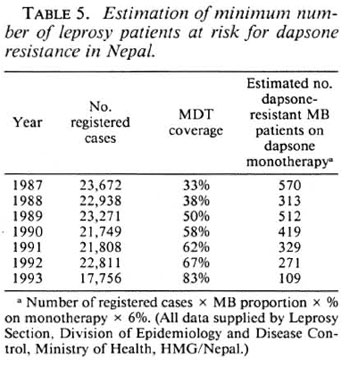
Primary dapsone resistance was found at a low level (6%) among new MB cases. Since the incubation period of paucibacillary (PB) leprosy is shorter, PB types of leprosy will tend to present earlier than MB types in people infected at the same time. Hence, we may expect a higher prevalence rate of primary dapsone resistance among PB patients than among the tested group of MB patients. Even if only 6% of new MB cases have dapsone resistance it means that in Nepal in the past 5 years hundreds of MB patients probably have received inadequate therapy and are potentially still infectious, if they have had dapsone monotherapy although being infected with dapsone-resistant organisms (Table 5). One previous report (l3) of primary dapsone resistant in Nepal is available. Between 1980-1982, Samuel, et al. found apparent resistance in 13 out of 15 new MB cases (87%). However, these were from a highly selected population, the majority being long-term residents of a government leprosarium, whereas in the current study the patients were likely to be more typical of the general population since the majority were from rural villages. In the scries by Samuel, et al. criteria for considering the MFP experiments to be positive were much less stringent than in our series.
Studies elsewhere have shown similar levels of primary dapsone resistance, e.g., 3.5% in The Philippines (4) and 6% in Cuba (3).
From cases tested for suspected secondary dapsone resistance between 1987 and 1992, 47% of the isolates were proven to be dapsone resistant. These patients had remarkably similar histories: low-dose dapsone monotherapy (equivalent to adult dose <50 mg/day) for several years, followed usually by a few years of full-dose dapsone (equivalent to 100 mg/day in an adult), then a treatment-free interval during which the patient was asymptomatic. Many other patients have had similar regimens but are now lost to follow up. Since any patients who have developed secondary dapsone resistance may be infectious long before they notice the clinical signs of relapse (reactivation), they are likely to infect others with dapsone-resistant bacteria. Hence, we may expect an increase in the prevalence of primary dapsone resistance in the future, although the time scale of this study is too short to demonstrate a secular trend.
It is no longer recommended practice in Nepal to undertake routine follow up of patients after release from treatment. Therefore a patient's reactivated disease usually will not be detected bacteriologically before he has overt skin lesions [as happened with our one relapse case (1759) who was detected bacteriologically at a routine surveillance visit 8 years after release from treatment]. At the time of stopping treatment, the patient must be made aware of warning signs and encouraged to report back to the clinic at his first suspicion of reactivation of disease.
One earlier report (14) of secondary dapsone resistance in Nepal indicated that 72.5% of treated MB cases may be dapsone resistant. This estimate was derived from a small number of cases (56 patients) most of whom were residents of Khokana Leprosarium. The sample was, therefore, biased toward people with more severe disease of longer duration. In addition to these considerations, the increasing use of MDT over the past 10 years may have resulted in a fall in prevalence of dapsone resistance.
All of the patients with confirmed dapsone resistance reported in this study subsequently have received MB MDT as recommended by WHO(15). Three patients received prothionamide instead of dapsone, because of hypersensitivity to dapsone, and six received ofloxacin in addition to routine MB MDT (as part of a separate research study). At 1-6 years after biopsy, 14 are known to have made satisfactory progress (including seven who have been released from treatment). For six patients we have no recent information, and one patient (7120) has not shown a satisfactory response either clinically or bacteriologically, although she has received supervised MDT almost continuously as an inpatient.
Rifampin resistance in Nepali leprosy patients has not been confirmed in any of our cases, and has not yet been reported by others.Continued screening of M. leprae isolates in a small number of laboratories is desirable to provide sentinel monitoring for drug resistance in endemic countries.
Acknowledgment. This work was supported by The Leprosy Mission International. We thank Dr. M. J. Colston of the National Institute of Medical Research, U.K., for the provision of rifampin in early studies; Dr. M. F. R. Waters for his advice, and Dr. S. Lucas for histology reports. Our thanks also go to the staff of Anandaban Leprosy Hospital, in particular, to Mr. Dhurba Mahat, Mr. Murari Thapa, Mr. Arjun Karki and Mr. Ishwor Shrestha in the laboratory, and Mrs. Sita Udas for typing the manuscript. We are also grateful to all of the patients who cheerfully cooperated in the study. This study was first presented at the XlVth International Leprosy Congress, Orlando,1993, but additional data have now been incorporated.
REFERENCES
1. BAQUILLON, G., FERRACI, C, SAINT ANDRE, P. and PATTYN, S. R. Dapsone resistant leprosy in a population of Bamako (Mali). Lepr. Rev. 51(1980)315-319.
2. BAQUILLON, G., FERRACI, C, VAN LOO, G. and PATTYN, S. R. Further results on dapsone resistant leprosy in Bamako (Mali). Lepr. Rev. 54(1983)19-21.
3. GONZALEZ, A. B., HERNANDEZ, C, SUAREZ, O., GONZALES-ABREU, E. and RODRIGUEZ, J. E. Survey for primary dapsone resistance in Cuba. Lepr. Rev. 57(1986) 341-346.
4. GUINTO, R. S., CELLONA, R. V., FAJARDO, T. T. and DELA CRUZ, E. C. Primary dapsone resistant leprosy in Cebu, Philippines. Int. J. Lepr. 49(1981)427-430.
5. MEADE, T. W., PEARSON, J. M. H., REES, R. J. W. and NORTH, W. R. S. The epidemiology of sulphone resistant leprosy. (Abstract) Int. J. Lepr. 41(1973)684.
6. NEELAN, P. N., NOORDEEN, S. K., BALAKRISHNAN, S. and RAJASEKARA PANDIAN, P. Prevalence survey of secondary dapsone resistance in leprosy in Kancheepuram and Tiruvanamal control units of Tamil Nadu. Lepr. India 55(1983)222-230.
7. PATTYN, S. R., YADA, A., SANSARRICO, H. and VAN Loo, L. Prevalence of secondary dapsone resistant leprosy in Upper Volta. Lepr. Rev. 55(1984)361-367.
8. PEARSON, J. M. H., REES, R. J. W. and WATERS, M. F. R. Sulphone resistance in leprosy, a review of one hundred proven clinical cases. Lancet 2(1975)69-72.
9. PETTIT, J. H. S. and REES, R. J. W. Sulphone resistance in leprosy, an experimental and clinical study. Lancet 2 (1964)673-674.
10. PETTIT, J. H. S., RIDLEY, D. S. and REES, R. J. W. Studies on sulphone resistance in leprosy. Int. J. Lepr. 34(1966)379-390.
11. REES, R. J. W. Drug resistance of Mycobacterium leprae, particularly to dapsone. Int. J. Lepr. 35(1967)625-638.
12. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity: a five-group system. Int. J. Lepr. 34(1966)255-273.
13. SAMUEL, N. M., SAMUEL, S., LOUDON, J. and ADIGA, R. B. Primary dapsone resistance in Nepal. Indian J. Lepr. 56(1984)819-822.
14. SAMUEL, N. M., SAMUEL, S., LOUDON, J., NEUPANE, K. and ADIGA, R. B. Prevalence of secondary Resistance in Nepal dapsone resistance in Nepal. Indian J. Lepr. 56(1984)823-827.
15. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
16. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.R.C.G.P.; Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
2. Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
3. M.Sc.; Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
4. B.Sc; Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
5. Ph.D., F.R.C.P.; Department of Medicine, University of Sydney, Sydney Australia.
Received for publication on 17 May 1995.
Accepted for publication in revised form on 14 December 1995.