- Volume 64 , Number 2
- Page: 97–104
Results ot a surveillance system for adverse effects in leprosy's WHO/MDT
ABSTRACT
The implementation of the World Health Organization's multidrug therapy (WHO/ MDT) in Brazil began slowly and gradually in 1986, and in 1991 it was adopted officially by the Brazilian Ministry for Health. After 1991, during the intensive phase of WHO/MDT implementation, there was some concern about the number of cases of renal failure observed in several Brazilian states, including some fatalities. This was the motive behind the state of São Paulo's Health Department's decision to carry out a study that would evaluate not only the incidence rate of adverse effects of rifampin in relation to kidney function but also in relation to the use of WHO/MDT in general.Due to the existence in the state of São Paulo of health services with a program for the control of Hansen's disease and an organized and stratified system of epidemiological surveillance, it was possible to elaborate a subsystem for data collecting. During the period f rom July 1991 to December 1993, 20, 667 patients were treated with WHO/MDT. Among this group there were 127 notifications considered as adverse effects, mainly:"flu"-like syndrome (54), acute renal failure (20), cutaneous reactions (15), toxic hepatitis (15), gastrointestinal complaints (8), hemolytic anemia (6), methemoglobinemia (4), thrombocytopenic purpura (2), hypotension (2) and disseminated intravascular coagulation (1). There was a predominance of adverse effects among multibacillary (MB) patients and the majority of the reactions occurred before the 6th dose; 82.7% of MB patients had had previous treatment with dapsone and rifampin and, due the fact that most severe reactions were related to rifampin, a booster mechanism could be an explanation for this occurrence. So far, there are seven published reports on renal failure in the world, and in Brazil only in the state of São Paulo there were 20 cases reported among 20, 667 patients under WHO/MDT treatment. This striking difference deserves a better explanation, but in no way do thèse reports undermine the positive aspects of WHO/MDT. However, the authors believe that a world alert about its possible serious side effeets is not only necessary but ethically required.
RÉSUMÉ
L'application de la polychimiothérapie de l'Organisation Mondiale de la Santé (PCT/OMS) commença doucement et graduellement au Brésil en 1986, et en 1991 elle a été officiellement adoptée par le Ministère de la Santé brésilien. Après 1991, durant la phase intensive d'application de la PCT/OMS, il y eut quelque inqiétude quant au nombre de cas d'insuffisance rénale, y compris quelques décès, observés dans plusieurs Etats brésiliens. Ceci a été le motif de la décision du Département de la Santé de l'Etat de Sao Paulo de conduire une étude qui évaluerait non seulement le taux d'incidence des effets secondaires de la rifampicinè en ce qui concerne la fonction rénale, mais aussi en ce qui concerne l'utilisation de la PCT/OMS en général.Du fait de l'existence, dans l'Etat de Sao Paulo, de services de santé avec un programme de lutte contre la maladie de Hansen et un système organisé et stratifié de surveillance épidémiologique, il a été possible d'élaborer un sous-système pour la collecte des données. Durant la période allant de juillet 1991 à décembre 1993, 20.667 patients ont été traités par PCT/OMS. Parmi ce groupe, il y eut 127 notifications de faits considérés comme effets secondaires, principalement: syndrome grippal (54), insuffisance rénale aiguë (20), réactions cutanées (15), hépatites toxiques (15), plaintes gastro-intestinales (8), anémies hémolytique (6), methémoglobinémie (4), purpura thrombocytopénique (2), hypotension (2) et coagulation intravasculaire disséminée (1). Il y avait une prédominance des effets secondaires parmi les patients multibacillaires (MB), et la majorité des réactions sont survenues avant la sixième dose; 82.7% des patients MB avaient été antérieurement traités par dapsone et rifampicinè et, du fait que les réactions les plus sévères étaient associées à la rifampicinè, un mécanisme sensibilisateur pourrait être une explication pour cette survenue. Jusqu'ici, il y a eu sept notification publiées de cas d'insuffisance rénale dans le monde, et au Brésil, dans le seul Etat de Sao Paulo, il y a eu 20 cas notifiés parmi 20.667 patients sous PCT/OMS. Cette différence frappante mérite une meilleure explication, mais en aucune façon ces notifications ne déforcent-clles les aspects positifs de la PCT/OMS. Cependant, les auteurs pensent qu'un monde attentif à ses possibles effets secondaires sérieux n'est pas seulement nécessaire mais éthiquement requis.
RESUMEN
La implementación de la poliquimioterapia propuesta por la Organización Mundial de la Salud (PQT/ OMS) en Brasil comenzó lenta y gradualmente en 1986; para 1991 la PQT fue adoptada oficialmente por el Ministerio Brasileño de Salud. Después de 1991, durante la fase intensiva de implementación de la PQT, hubo cierta preocupación por el número de casos que presentaron algún tipo de falla renal, incluyendo algunas muertes, en varios estados Brasileños. Esto motivó al Departamento de Salud del estado de Sao Paulo a estudiar la incidencia de efectos adversos sobre la función renal de la rifampina y de la PQT/OMS en general. Gracias a que en el estado de Sao Paulo existen programas bien organizados para el control y la vigilancia epidemiológica de la enfermedad de Hanscn, se pudo elaborar un subsistema para la colección de datos. Durante el periodo de julio de 1991 a diciembre de 1993, se trataron 20,667 pacientes con la PQT/OMS. En este grupo hubieron 127 notificaciones sobre efectos adversos que incluyeron: un síndrome parecido a la influenze (54 casos), fall renal aguda (20), reacciones cutáneas ( 15), hepatitis tóxica ( 15), molestias gastrointestinales (8), anemia hemolítica (6), metahcmoglobinemia (4), púrpura trombocitopénica (2), hipotensión (2) y coagulación intravascular diseminada (1). Los efectos colaterales predominaron entre los pacientes multibacilares (MB), y la mayoría de las reacciones ocurrieron antes de la sexta dosis. Dado que el 82.7% de los pacientes MB habían recibido tratamiento previo con dapsona y rifampina y debido a que las reacciones más severas estuvieron relacionadas con la rifampina, es probable que su ocurrencia se haya debido a un efecto"booster." Hasta ahora existen 7 publicaciones sobre falla renal en el mundo, y en Brasil sólo en el estado de Sao Paulo hubieron 20 casos reportados entre los 20,667 pacientes sujetos a la PQT/OMS. Esta gran diferencia requiere una mejor explicación pero este reporte de ninguna manera demerita los aspectos positivos de la PQT/OMS. Sin embargo, los autores piensan que una llamada de alerta a nivel mundial sobre sus posibles efectos colaterales no solo es necesaria sino también éticamente requerida.At the moment, the best available drugs for the treatment of Hansen's disease are: dapsone, clofazimine and rifampin which, together, form the multidrug therapy (MDT) scheme highly recommended by the World Health Organization (WHO).
Dapsone was first used to treat this disease in the beginning of the 1940s, clofazimine in 1963 and rifampin in the beginning of the 1970s. As with all drugs, collateral effects or side effects are to be found. However, in the case of these drugs, the frequency of such collateral effects has never been such as to warrant a halt to their widespread use.
Dapsone can present a number of adverse effects: hemolytic anemia, methemoglobinemia, exfoliative dermatitis, photosensitive drug reactions, jaundice, psychotic reactions and dapsone syndrome.
Clofazimine frequently causes ichthyosiform scaling, changes in the color of the skin, the mucous membranes, the urine, and the respiratory secretions. The reddishbrown pigmentation, which appears after the patient has been using this medication for some time, and which is more evident in the cutaneous lesions, disappears slowly when the drug is stopped. The drug also can cause diminished peristalsis and abdominal pain caused by crystals depositing in the walls of the bowel. The high dosages (200 mg-300 mg) used to treat leprosy reactions can lead, in some cases, to intense abdominal cramping which can simulate a case of acute intestinal obstruction.
Rifampin can provoke cutaneous eruptions, thrombocytopenic purpura, hepatitis, a flu-like syndrome, hemolytic anemia, shock, respiratory insufficiency and acute renal failure caused by interstitial nephritis or acute tubular necrosis. The most serious adverse effects of this drug result from its intermittent use, as was observed in the treatment of tuberculosis with weekly dosages (or sometimes once or twice a week) (3.8,15).
In 1981, WHO introduced its multidrug therapy using these three drugs which are considered to be the best. Although some authors (13,14)- remark that with this new chcmotherapcutic regimen the incidence of adverse effects in relation to dapsone is more frequent, including a possible rise in the dapsone syndrome, other authors do not report any increase in the incidence rate of these effects. There is also no increase in the incidence rate of these reactions for either of the other two drugs.
Brazil was one of the first countries to adopt a combination of drugs in the treatment of Hansen's disease. Rifampin and dapsone have been used since the end of the 1970s to treat borderline and lepromatous patients (dapsone 100 mg per day to clinical cure and 600 mg of rifampin per day for 3 months). In cases of dapsone resistance, clofazimine (100 mg per day) was used instead of dapsone. Paucibacillary cases were treated only with dapsone. The incidence of adverse effects with this regimen was no greater than that observed when these drugs are used individually.
The implementation of the WHO/MDT in Brazil began slowly and gradually in 1986, and in 1991 it was adopted officially by the Ministry for Health, substituting for the previous regimen (Ministério da Saúde. Fundação Nacional de Saúde. Avaliação independente do programa de controle e elminiação da hanseniase. Relatório final. Brasilia, Nov. 1992).
After 1991, however, during the intensive phase of the WHO/MDT implementation, there was some concern about the number of cases of renal failure observed in several Brazilian states, including some fatalities. In fact, in some places the number of cases of acute renal failure was such that staffs of several health centers questioned whether or not they should continue to administer WHO/MDT. This was also the motive behind the state of São Paulo's Health Department's decision to carry out a study that would evaluate not only the incidence rate of adverse effects in relation to kidney function and rifampin but, also, in relation to the use of WHO/MDT in general. It is also the reason for the presentation of this paper.
MATERIALS AND METHODS
Since there already exists throughout the state of Sao Paulo a network of health services with a program for the control of Hansen's disease and an organized and stratified system of epidemiological surveillance, it was possible to create a subsystem for collecting data on the possible adverse effects of WHO/MDT. The epidemiological surveillance system is organized into three levels, according to the area covered and its responsibilities, i.e., a central level, the Epidemiological Surveillance Center (with state responsibilities), a regional level, the Regional Health Offices (with regional responsibilities), and the local level, the Health Unit (with municipal responsibilities).
To facilitate the study, a special form was designed for collecting data of possible adverse effects. A data flow system was also organized between the different levels of the health systems and, finally, a referral system was also provided to cope with more complicated cases.
Through the Regional Health Offices, the 550 Health Units (where the WHO/MDT was administered) were forewarned about the possible adverse effects of WHO/MDT and also briefed on the procedures to be adopted in dealing with these cases and their respective notification.
The form requested general data referring to Hansen's disease, as related below, from the type of treatment given to the undesirable effects,presented: 1) Identification (personal data, clinical condition, previous and present treatment for leprosy, date when the treatment began). 2) Laboratory tests, if any, that were made prior to the beginning of WHO/MDT, such as: serum transaminase level, alkaline phosphatase, bilirubin, plasma creatinine, complete blood count, urinalysis and any test made due to problems other than leprosy. 3) Personal history-use of other medicines not related to the treatment of leprosy, of any concomitant disease and history of allergic reactions to drugs. 4) Family history. 5) Data relating to adverse effects, such as: diagnosis, duration, evolution, laboratory findings, treatment given for the adverse effect, hospitalization, reintroduction or not of some kind of leprosy therapy and what this consisted of. This data was provided directly by the Health Unit or hospital staff or collected from the patient records.
Each notification was checked to see if the diagnosis of the adverse effect was coherent with the clinical description and the results of the laboratory tests that were done. A special data flow on adverse reactions was set up alongside the regular communication flow of the epidemiological surveillance. The initial notification was to be given immediately, by phone or fax, to the central level so that support could be sent to the Health Unit with regard to any difficulties in dealing with these cases. This direct line was also used to guarantee that the forms were filled in with all the relevant data available.
Each Regional Health Office was responsible for investigating the notification, organizing the hospital and laboratory to which the patients should be referred and, when necessary, for facilitating the communication between the local and central levels.
The State Reference Centers (i.e., Lauro de Souza Lima's Research Institute for the interior of the state and the Reference Clinic for Hansen's Disease for the Metropolitan Area of São Paulo) gave the necessary orientation and, in the case of the Lauro de Souza Lima's Institute, conditions for hospitalization as well. The completed forms were sent to the central level to have the data analyzed according to clinical and laboratory criteria, to confirm the diagnosis and, later, they were processed using the EPI Info 5,.lb microcomputer program.
RESULTS
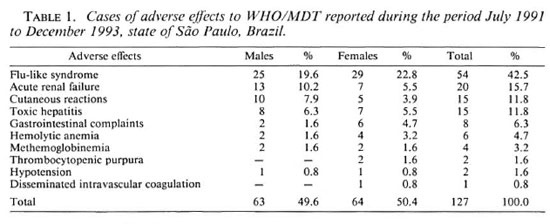
In the state of São Paulo during the period starting with the implementation of WHO/ MDT for Hansen's disease from July of 1991 until December of 1993, 20, 667 patients were treated with this therapy. Among this group there were 211 notifications of adverse effects received during this period. Out of these, 127 were considered as adverse effects and the remaining 84 were discarded, either because the data presented were considered incomplete or the diagnosis presented was compatible with manifestation of the disease itself (e.g., erythema nodosum leprosum or neuritis).
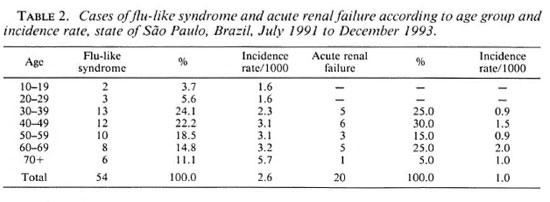
Of the 127 cases of adverse effects, 43 were hospitalized. The only cases to be grouped according to age were those involving a flu-like syndrome and acute renal failure (Table 2) because of their higher number and close relation to rifampin use.
With regard to the cutaneous reactions found, there were: 2 cases of erythema multiforme, 1 facial erythema, 2 exanthematous reactions, 2 erythrodermas, 2 photosensitive reactions, and 6 urticarias.
Gastrointestinal conditions were made up basically of five cases of gastric intolerance and two abdominal complaints caused by clofazimine.
The proportion of paucibacillary (PB) cases included in the WHO/MDT during the period studied was 31.0% (6410) and that of multibacillary (MB) cases was 69.0% (14, 257). The number of PB cases presenting adverse effects made up 18.1% (23) of the total number of adverse effects reported, while MB cases made up the other 81.9% (104).
All of the cases of acute renal failure had a prior history of specific treatment and all were MB cases. Of the 54 cases of flu-like syndrome, 47 had had previous treatment and of these 43 were MB cases.
In the group that had had no previous treatment, there were 7 cases of flu-like syndrome, 6 hepatitis, 2 hemolytic anemia, 2 methemoglobinemia, and 1 case of acute abdominal syndrome; the remainder were made up of various cutaneous allergies. Of the seven cases of flu-like syndrome with no prior treatment, six were MB cases.
The incidence rate of adverse effects among MB cases who had had previous treatment was 7.3 per 1000 treated cases; in PB cases the rate was 3.6 per 1000 cases.
The relationship between the number of supervised dosages and the manifestation of the flu-like syndrome varied from the 1st to the 18th dose, the average being 5.6 doses. The vast majority of acute renal failure cases occurred between the 2nd and 5th supervised dose. Hepatitis came on mainly between the 1st and 4th dose. The case of disseminated intravascular coagulation occurred at the 3rd dose. Considering all of the 127 cases as a whole, 95 ofthem (74.8%) presented undesirable effects before or up to the 6th dose. In relation to the supervised dose and the onset of the adverse effect, no difference was observed between those who had been treated previously for Hansen's disease and those who were newly diagnosed patients.
In relation to the progress of the patients, 104 advanced toward cure, 21 cases were still under observation while the data were being collected, and 2 patients had died (1 due to acute renal failure, the other due to disseminated intravascular coagulation).
Of the total 127 cases, 100 (78.7%) were re-introduced to treatment with alternative regimens which excluded those drugs suspected of causing the undesirable reactions. Four cases were considered cured of leprosy and released, while the remaining cases either continued under observation or the patient himself refused further treatment.
DISCUSSION
The number of Health Units in São Paulo state where WHO/MDT is used represent a population coverage of 95.0%. The majority ofthese Units also have the services of medical doctors to treat their patients. Among the accumulated number of patients treated with WHO/MDT between July 1991 and December 1993 (20, 667), 7020 were newly diagnosed cases and 13, 647 were patients who had already been undergoing specific treatment. The cases of adverse effects analyzed here occurred within this accumulated number of patients under WHO/MDT.
During the process of analyzing each notification, care was taken to evaluate the reliability of the collected data and to verify whether the clinical description of the adverse effect was compatible with the laboratory tests. In the same way, the maximum amount of data was gathered to establish, as rigorously as possible, a causal relationship between the adverse reaction and the suspected drug. In this type of study, the amount and quality of the data are fundamental, not only to establish correlations of this nature but, also, to fulfill the requirements for publication of such material.
Laporte and Lience (7), addressing this subject, said that in examining the notification of isolated cases of adverse effects that are to be submitted for external inspection, it is essential that they contain information on sex, age, the suspected drug and all drugs taken simultaneously, including information on when they were taken, the dosage and the way they were administered, the reason they were prescribed, the time sequence between the administration of the drug and the onset of adverse effect. They also stress the importance of giving information on clinical progress, and any other health problems or relevant environmental factors, with corresponding data, as well as the patient's previous history of adverse effects to any other similar type drugs. They also recommended that previous publications about similar cases, if any, be mentioned, and any other factors that might be relevant to confirm some specific reactions (for example: blood concentrations, laboratory information, history and ethnic background). In presenting our data, we tried to follow these steps.
Of the 211 cases of adverse effects that were reported, 84 were discarded. Of these 84 cases, a considerable number concerned a diagnosis of erythema nodosum leprosum and neuritis, which were misinterpreted as adverse effects of the medications.
Even with the ongoing and wide-scale training that was given to all the Health Units, there were still a number of professionals who confused some of the features of the disease with collateral phenomena. The reason for this incorrect diagnosis could be that the training given at the health centers failed to reach some professionals due to the high rate of turnover. In Brazil, this kind of turnover is a direct consequence of the low salaries offered. For this reason, there is a certain lack of pace between the training and the replacement of personnel. This would also explain the unexpected, probably low, number of notifications of adverse reactions, especially those of the oligosymptomatic nature. It was impossible to estimate the number of these unreported cases which, in turn, impaired to some extent the analysis of the specific incidence rates referring to age, sex and clinical type of Hansen's disease. Table 2 shows the progressive increase in the incidence rate of the flu-like syndrome according to age groups. Perhaps the reason for this is that the older age groups go to Health Units more often, even when their symptoms are mild, and the opposite is often true for younger people.
Acute renal failure cases (Table 2) were predominantly in those over 30 years of age, but for these people the age factor seems to be irrelevant.
Another difficult factor to evaluate was the occurrence of simultaneous diseases, such as diabetes or hypertension, since the incidence rate of these conditions among leprosy patients under treatment was unknown. Thus, it was not possible to determine the relationship between these pathologic conditions and the adverse effects.
There was a predominance (Table 3) of adverse effects among MB patients. With the exception of nine cases of the flu-like syndrome which presented its first manifestations between the 6th and the 18th doses, the majority of the reactions occurred before the 6th dose. The fact that MB cases continued treatment for a longer period than did the PB cases, presented no noticeable difference. Both presented most of the undesirable reaction before the 6th month.
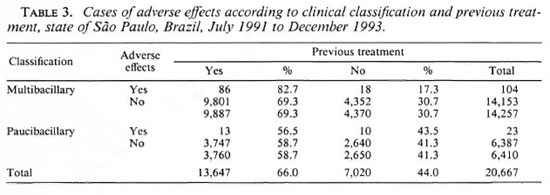
The majority of MB patients who presented with adverse effects (86 out of 104, 82.7%) had had previous treatment with dapsone and rifampin, and 6 of the 23 PB patients had been treated previously with only dapsone. In the same way, the most severe cases occurred among the MB patients who had received previous treatment (73.3% of them consisted of flu-like syndrome and acute renal failure).
The adverse effects among the PB cases were mostly benign, with diagnosis compatible with reactions possibly related to dapsone, such as: erythema multiforme (1), gastric complaints (3), methemoglobinemia (2), hemolytic anemia (1), urticaria (3), exfoliative dermatitis (2) and photosensitive reactions (1). There were also four cases of toxic hepatitis which could have been caused by dapsone or by rifampin. Some of the cases are evidently related to rifampin, such as a case of facial erythema and five cases of flu-like syndrome.
The MB cases presented symptoms suggesting a rifampin etiology, such as: acute renal failure (20), flu-like syndrome (49), and thrombocytopenic purpura (2). The other conditions could have been caused either by rifampin or by dapsone [toxic hepatitis (11), hemolytic anemia (5)], and the rest were distributed between gastric complaints and cutaneous reactions.
The most severe reactions, without a doubt, were related to rifampin. A certain number of these reactions seem to Delinked to the formation of immune complexes. It was seen repeatedly that the intermittent administration of an antigenic drug more frequently caused adverse reactions than when it was administered daily, and this could be the case with rifampin. The higher frequency of reactions with intermittent treatment is linked to the fact that the administration of an antigen in weekly doses represents a booster mechanism, which is reasonably good for sensitization (l6). In this way, it could explain the adverse effects of rifampin administered once a month in Hansen's disease, in the same way as that used in tuberculosis once or twice a week. The majority of our cases presenting with serious reactions had been treated previously with the combination of rifampin and dapsone. This higher incidence among these cases also could be explained by the booster mechanism. A reason for the reactions to happen right at the beginning of treatment could be that the first dose of WHO/MDT functioned as a booster due to the rifampin. Any possible interaction between rifampin, dapsone and clofazimine docs not seem to have any relevant significance in the circumstances.
With the exception of renal failure, there was no evidence among our cases that the adverse effects of WHO/MDT were any higher than those observed when the drugs are used separately. It was really the information received about renal failure that inspired the undertaking of this study and, in fact, the number of patients presenting with this adverse effect was indeed much higher than expected. A recent study of published reports on renal failure attributed to the use of WHO/MDT documented seven cases (l0) and six cases (2,12).
In Brazil, we are aware of some cases of this nature, but the only published report refers to five patients in Londrina in the state of Parana (4). In our study, which refers only to the state of São Paulo, 20 cases were reported among 20, 667 patients under WHO/MDT. All of the other reported cases come from India, and although Brazil and India have the highest number of registered patients in the world, it is difficult to believe that other countries have no reported cases of renal lesions with WHO/MDT. Likewise, it is difficult to explain why the number of these cases in Brazil is greater than in India, where the number of patients under WHO/ MDT is much higher (1,5,6,11).
One explanation could be the"booster" effect in those patients who have been treated previously with rifampin. Another possibility, put forward by Opromolla, could be the fact that in most of the control programs for Hansen's disease in Brazil, it is the physician who is responsible for examining the patient and following up the case. This makes the detection of any possible renal complications more likely. The later is further evidenced by the fact that in the state of Amazona, where most of the program is carried out by paramedical workers, there was only one reported case of renal failure and this case was diagnosed in the capital, Manaus, by a physician.
If our findings about the adverse effects are acknowledged, and our explanation for their higher incidence rate in Brazil is accepted, it is of extreme importance that the real number of these cases should be verified in the rest of the world. We are certain that this in no way undermines the positive aspects of WHO/MDT, but we believe that a worldwide alert about possible serious side effects is not only necessary but ethically required.
Acknowledgment. We are grateful to Dr. Marcos Virmond and to: Tanya Eloise Lafratta, Therese A. Araujo, Dalila Mohallen, Marcia Buzzar, Marli Manini and all Health Units and Regional Offices that participated in this surveillance system.
REFERENCES
1. Dedhia, N. M., Almeida, A. F., Khanna, U. B., Mittal, B. V. and Acharya, V. M. Acute renal failure: a complication of the new multidrug regimen for treatment of leprosy. Int. J. Lepr. 54(1986)380-382.
2. Ekambaran, V. and Rao, M. K. Changing picture of leprosy in North Arcot District, Tamil Nadu, after MDT. Indian J. Lepr. 61(1989)31-34.
3. Girling, D. J. Adverse reactions to rifampicin in antituberculosis regimens. J. Antimicrob. Chemother. 3(1977)115-132.
4. Gordan, P. A., Grion, C. M. C, Sousa, V., Carvalho, V. P., Delfino, V. D. A., Mendes, M. F., Matini, A. M. and Mocelini, A. J. Insuficiencia renal aguda pelo uso do esquema multidroga no hanseniase. Hansen. Int. 17(1992)21-26.
5. Gupta, A., Sakhuja, V., Gupta, K. L. and Chugh, K. S. Intravascular hemolysis and acute renal failure following intermittent rifampin therapy. Int. J. Lepr. 60(1992)185-188.
6. Kar, H. K. and Roy, R. G. Reversible acute renal failure due to monthly administration of rifampin in a leprosy patient. Int. J. Lepr. 54(1984)835-839.
7. Laporte, J. R. and Lience, E. Información minima que deben contener las publicaciones sobre sospechas de reacciones adversas a medicamentos. Med. Clin. (Bare.) 97(1991)56-57.
8. Morrone, N., Feres, W. J. M. and Fazolo, N. Efeitos colaterais dos tuberculostaticos. Rev. Bras. Clin. Terap. 11(1982)212-225.
9. Noordeen, S. K. Worldwide implementation of WHO/MDT and experiences gained (Conference on Chemotherapy of Leprosy, Greenville, South Carolina, U.S.A., 6-8 April 1992). Geneva: World Health Organization, 1992.
10. Opromolla, D. V. A. Adverse reactions to rifampicin with special reference to acute renal failure. (Editorial) Hansen. Int. 17(1992)1-4.
11. Palande, D. D. La polychimiothérapie entrainet-elle des complications? (Editorial) Acta Leprol. 7(1990)105-107.
12. Ramu, G. Problems of multidrug therapy. Indian J. Lepr. 63(1991)435-445.
13. Reeve, P. A., Ala, J. and Hall, J. J. Modification of multidrug treatment of leprosy in Vanuatu. Int. J. Lepr. 60(1992)655-656.
14. Richardus, J. H. and Smith, T. C. Increased incidence in leprosy of hypersensitivity reactions to dapsone after introduction of multidrug therapy. Lepr. Rev. 60(1989)267-273.
15. Riska, H. and Tonroth, T. Nefritis tubulo-intersticial aguda después de un tratamiento con rifampicina. Bull. Union Int. Tuberc. 59(1984)148-151.
16. Stevens, E., Joniau, M., Verbist, L. and Saerens, E. Immune complexes in rifampicin side reactions. Symposium on Rifampicin in Tuberculosis Treatment, Helsinki, 22-23 February 1974.
1. M.D., M.Sc; Leprosy Surveillance Service, State of São Paulo, Av. São Luis 99, 6 andar, São Paulo 01046001, SP, Brazil.
2. M.D; Leprosy Surveillance Service, State of São Paulo, Av. São Luis 99, 6 andar, São Paulo 01046001, SP, Brazil.
3. M.D., Ph.D.; Chief, Division of Research and Training, Instituto Lauro de Souza Lima, Bauru, SP, Brazil.
4. M.D.; Chief, Leprosy Control Program, State of São Paulo, São Paulo, SP, Brazil.
Reprint requests to Dr. Opromolla.
Received for publication on 11 December 1995.
Accepted for publication on 17 January 1996.