- Volume 64 , Number 2
- Page: 173–5
TT leprosy: does it indicate percutaneous infection?
To the Editor:
The subepidermal zone (SEZ) is a narrow strip of dermis which lies just below the basal layer of the epidermis. Histological changes in the SEZ find mention even in the early literature, and the SEZ is regarded as a differentiating point between tuberculoid and lepromatous leprosy. A free SEZ is also know as the band of Unna, who first stated that this zone is free of bacilli when compared to the lepromatous granuloma (12). Complete obliteration of the SEZ by epithelioid cell granuloma is exclusively a feature of tuberculoid (TT) leprosy as per the Ridley-Jopling classification (7). Hence, this piece of histologic change isolates TT from almost all other types of leprosy. While we have some convincing explanation about the free grenz zone of lepromatous leprosy (4), the significance of its obliteration in TT leprosy is not adequately explained. Evolution of a granuloma in the most superficial part of the dermis makes more sense as a starting point of the granuloma and, more importantly, might indicate the cutaneous route of infection.
Although inhalation of bacilli-rich droplets is at present regarded as the most common mode of entry by leprosy bacilli (3), infection through the skin, as advocated in earlier studies (1,2,11), has not been discarded. Can the evolution of TT leprosy be attributed to the percutaneous entry of the bacilli? Will the route by which the antigen gains access to the biological system decide the nature of the immune response? An important study by Ridley (5) states that granuloma in the SEZ and the epidermis is found in cases with high resistance which detects and mounts an immune attack at the site of entry of Mycobacterium leprae. Now there are pieces of evidence which attribute an antigen-processing function to the epidermal Langcrhans' cells in many delayed-type hypersensitivity (DTH)-mediated diseases, including leprosy (10). These mononuclear phagocytes are known to process and transport M. leprae antigens through the subepidermal lymphatics to regional lymph nodes and lymphocytes on the route for an effective immune response (8,9).
On the basis of these observations, one is tempted to interpret the pathogenesis of TT and other types of leprosy mainly around the route of infection. If the organism is detected by the antigen-processing cells, there is an effective immune response, resulting in a localized TT leprosy. If this check system is bypassed due to entry through routes other than the skin, an entirely different course of the disease may ensue. An epithelioid cell granuloma, including that of TT, is a manifestation of DTH. We cannot think of its evolution without a phase of acute inflammation. Type 1 reaction involving the SEZ and the epidermis appears to represent this acute state. In other words, TT cases with involvement of both the SEZ and the epidermis may have started out with a type 1 reaction although they are now immunologically stable, i.e., TT patients may have started out as borderline. This also may explain the occurrence of type 1 reaction, which is a strong borderline feature, in some TT cases which as a whole are considered immunologically stable. The apparent low lymphocyte density and the lack of focalization (Fig. 1) were due to massive inflammatory edema. These reacting granulomas probably indicate a recent sensitization and a persisting antigenic stimulation. As the acute phase subsides, the granuloma attains the compact histology usually described forTT leprosy. It is logical to suppose that in TT leprosy DTH follows anacutc-to-chronic course. The histology conventionally described is that of the chronic state with shrinkage of the granuloma and an apparent increase in lymphocytes (Fig. 2) due to drainage of the inflammatory exudate. There is loss of giant cells and epithelioid cells, probably due to their shorter life span (6).
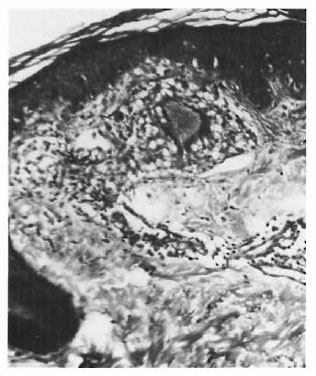
Fig. 1. Acutely inflamed granuloma in the subepidermal zone (SEZ).
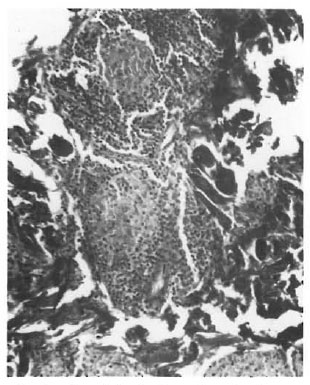
Fig. 2. Chronic focalized granuloma showing nestsof epithelioid cells with a cuff of lymphocytes.
The foregoing discussion proposes that the granuloma in TT leprosy develops from without due to cutaneous infection and most lesions probably indicate the site of inoculation. Lara and Nolasco (3) state that at the uppermost tuberculoid side of the disease spectrum a group of vaccination lesions, including self-healing childhood leprosy, is recognized. "... It is probable that most of them are benign, but there is always the possibility that they and other undetected forms...may serve as the sources for at least some apparently new infections occurring in older children and adults...."
It is the general impression that knowledge regarding the pathogenesis of tuberculosis has considerable influence on the understanding of leprosy. But an equivalent of the primary complex in leprosy is not yet defined. Localized skin lesions, the tendency to self-healing and characteristic histology, all make TT leprosy a closer candidate. Lara and Nolasco's quote above sounds like primary and secondary leprosy.
- Dr. D. Porichha
Senior Pathologist
Medical Centre
Parliament House Annexe
New Delhi 110001, India
REFERENCES
1. Horton, R. J. and Povey, S. The distribution of first lesions in leprosy. Lepr. Rev. 37(1996)113-114.
2. Job, C. K., Selvapandian, J. A. and Kurian, P. U. Leprosy Diagnosis and Management. New Delhi: Indian Leprosy Association, 1975.
3. Lara, C. B. and Nolasco, J. O. Self healing, or abortive, and residual forms of childhood leprosy and their probably significance. Int. J. Lepr. 24(1956)245-263.
4. Martens, U. and Klingmuller, G. Free subepidermal grenz zone (band of Unna) in lepromatous leprosy; histological and ultrastructural findings. Int. J. Lepr. 52(1984)55-60.
5. Ridley, D. S. Pathology and bacteriology of early lesions in leprosy. Int. J. Lepr. 39( 1971 )216-224.
6. Ridley, D. S. The pathogenesis of skin lesions. In: Skin Biopsy in Leprosy. Basle: Ciba Gcigy Limited, 1977.
7. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-272.
8. Silberberg-Sinakin, I. On Langerhans' cells. Int. J. Dermatol. 16 (1977)581-583.
9. Silberberg-Sinakin, I., Thorbecke, G. J. and I3aer, R. L. Langerhans' cells in skin, dermal lymphatics and in lymph nodes. Cell. Immunol. 25(1976)137-151.
10. Stingle, G., Tamaki, K. and Katz, S. I. Origin and function of epidermal Langerhans' cells. Immunol. Rev. 53(1980)149-174.
11. Susman, I. A. A limited investigation into the significance of the site of first lesion in leprosy. Lepr. Rev. 38(1967)37-42.
12. Unna, P. G. Lepra. In: Histopathologic der Hautkrankheiten; Lehrbuch der spczillcn pathologischen Anatomie. Vol. 8, Chapter 2. Berlin: Verlag V. August Hirschwald, 1894, pp. 603-617.
13. World Health Organization. A guide to leprosy control. 2nd edn. Geneva: World Health Organization, 1988, p. 8.