- Volume 64 , Number 2
- Page: 142–5
Determination of the minimal effective dosages of ofloxacin and sparfloxacin against M. leprae in the mouse foot pad system
ABSTRACT
The minimal effective dosages (MEDs) of ofloxacin (OFLO) and sparfloxacin (SPFX) against 10 isolates of Mycobacterium leprae were measured in the mouse foot pad system. The drugs were administered either by gavage or by incorporation into the mouse diet in a range of concentrations. The results demonstrated that the MEDs of OFLO were 4 to 5 times higher than those of SPFX, thus confirming that, on a weight-to-weight basis, the anti-M. leprae activity of SPFX was significantly greater than that of OFLO. The MEDs of OFLO/SPFX measured by gavage were 20 times lower than those measured by incorporating the drug into the mouse diet.RÉSUMÉ
Les doses effectives minimales (DEM) d'ofloxacine (OFLO) et de sparfloxacinc (SPFX) vis-à-vis de 10 isolats de Mycobacterium leprae ont été mesurées dans le système du coussinet plantaire de la souris. Les médicaments ont été administrés, soit par gavage, soit par incorporation dans le régime alimentaire, à diverses concentrations. Les résultats ont démontré que les DEM d'OFLO étaient 4 à 5 fois plus élevées que celles de SPFX, confirmant donc que, à poids égal, l'activité anti-M. leprae de SPFX était significativement plus grande que celle de l'OFLO. Les DEM d'OFLO/SPFX mesurées par gavage étaient 20 fois plus basses que celles mesurées en incorporant le médicament dans le régime alimentaire.RESUMEN
Se midieron las dosis mínimas efectivas (DME) de la olloxacina (OFLO) y la esparlloxacina (SPFX) contra 10 aislados de Mycobacterium leprae en cl sistema de la almohadilla plantar del ratón, las drogas se administraron por sonda gástrica o incorporadas en la dicta a diferentes concentraciones. Los resultados demostraron que las DM Es de la OFLO fueron de 4 a 5 veces mayores que las de la SPFX confirmando así, que sobre la base de peso a peso, la actividad anti-M leprae de la SPFX fue significativamente mayor que la de la OFLO. Las DMEs de la OFLO y la SOFX administradas por sonda fueron 20 veces menores que cuando las drogas se incorporaron en la dieta del ratón.Fluoroquinolones inhibit the bacterial gyrase, a target which has never been exploited in leprosy chemotherapy. Since the introduction of rifampin (RMP), fluoroquinolone was the first lead to an important antileprosy drug in many years, and ofloxacin (OFLO) is one of the most active compounds against Mycobacterium leprae among commercially available fluoroquinolones (5,6,7,10). Because OFLO displayed strong bactericidal activity against M. leprae in mice (5) and in patients (6,10), the long-term therapeutic effect of a regimen with 1-month duration of daily OFLO + RMP for both paucibacillary (PB) and multibacillary (MB) leprosy is being tested in a multicenter field trial organized by the World Health Organization. Sparfloxacin (SPFX) is a newer fluoroquinolone, and is now commercially available in a number of countries. On a weight-to-weight basis, it has more potent in vitro (2) and in vivo (3,4) activity against M. leprae than OFLO. Nevertheless, to date, the manufacturer of SPFX only recommends a dosage of no more than 200 mg per day, about half or one-fourth the dosage of OFLO; thus, the greater activity of SPFX is likely to be offset by its lower clinical dosage. In fact, when the drugs were administered in mice at dosages corresponding (in terms of the area under the concentration-time curve) to their clinical dosages, the bactericidal activity of SPFX was very close to that of OFLO (9). The results of a clinical trial demonstrated that the therapeutic effect of SPFX 200 mg daily in lepromatous patients (1) also was very similar to that in patients treated by OFLO 400 mg daily in a separate trial (6). Therefore, the real advantage of SPFX over OFLO in the treatment of leprosy remains unclear.
Although OFLO or SPFX may be an important component of a newer generation multidrug regimen(s) for leprosy, the criteria for the diagnosis of OFLO- and SPFXresistant M. leprae have yet to be established by the determination of their minimal effective dosage (MED) against M. leprae in the mouse foot pad system (8). Furthermore, comparing the MEDs of OFLO and SPFX may provide more precise information about comparative in vivo activities against M. leprae between the two derivatives. .We have, therefore, determined the MEDs of OFLO and SPFX against 10 isolates of M. leprae in the mouse foot pad system by standard methods (8).
MATERIALS AND METHODS
Antimicrobial agents. OFLO and SPFX were generously provided, respectively, by Roussel Uclaf, Romainville, France, and Rhone D.P.C. Europe, Antony, France.
M. leprae All of the 10 M. leprae strains were isolated directly from multibacillary (MB) patients of Institut Marchoux. There were 6 males and 4 females between the age of 27 and 59 years; 7 lepromatous (LL) and 3 borderline lepromatous (BL) leprosy; 2 were previously untreated; the other 8 had relapsed after dapsone (DDS) monotherapy. None of them had been exposed previously to fluoroquinolones.
Mouse foot pad inoculation. Onc-hundred-seventy-six (176) mice were inoculated into both hind foot pads with M. leprae recovered from a skin biopsy obtained from each patient. The inoculum was 5 x 103 organisms per foot pad.
Drug administration. After inoculation, the 176 mice were divided into 17 groups: a control group of 16 mice without treatment and 16 treated groups of 10 mice each. Treatment began immediately after inoculation and continued until sacrifice. As shown in Tables 1 and 2, in each group treatment was administered by esophageal cannula (gavage) 6 times weekly with one of the four suspensions of either OFLO or SPFX, or were fed daily with one of the four mouse diets incorporated with different concentration of OFLO or SPFX. For gavage, the drug was suspended in 0.05% agar in distilled water at concentrations of 5, 2.5, 1.25, 0.625 and 0.313 mg/ml, which corresponded to 50, 25, 12.5, 6.25 and 3.13 mg/kg/dose when 0.2 ml suspension was administered for a mouse with a body weight about 20 g, or 0.3 ml for a mouse with a body weight of about 30 g. The drug suspensions were prepared every 2 weeks and kept at 4ºC. To prepare the drug-incorporated mouse diet, OFLO or SPFX was mixed with the mouse diet in as uniform a manner as possible (8) at concentrations of 0.5%, 0.25%, 0.1% and 0.05% (w/w) for OFLO and 0.1%, 0.05%, 0.025% and 0.01% (w/w) for SPFX. Assuming that a mouse with a body weight of 25 g consumed 5000 mg of diet every day, in mouse diet incorporated with OFLO/SPFX at concentrations 0.5%, 0.25%, 0.1%, 0.05%, 0.025% and 0.01% corresponded, respectively, to administering the drug at 1000, 500, 200, 100, 50 and 20 mg/kg/daily.
Harvests of M. leprae . Six months after inoculation, M. leprae were harvested by Shepard's method (13) from individual inoculated foot pads of three control mice, and repeated at intervals of 2 months until the average number of acid-fast bacilli (AFB) per foot pad was found to be at least 5 x 105 or at 12 months if the organisms were found to have multiplied (defined as harvested > 1 x 105 AFB/foot pad) in the control mice but fewer than the average of 5 x 105 AFB/foot pad. The foot pads of all surviving control and treated mice also were harvested.
Determination of MED. The MED is defined as the lowest drug concentration administered in the mice in which multiplication of M. leprae has not been observed in a single foot pad, while the multiplication is observed in a significant proportion of foot pads in control mice.
RESULTS
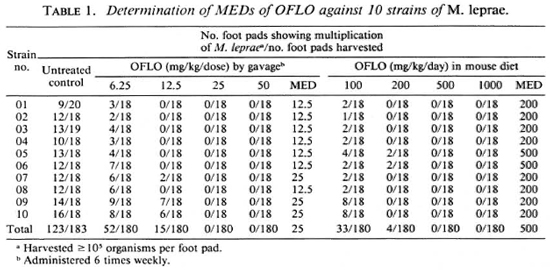
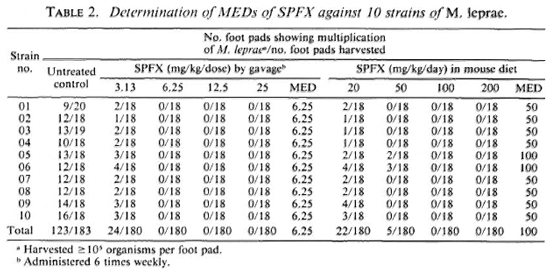
As shown in Tables 1 and 2, multiplication of M. leprae was observed in a significant proportion of untreated control mice, indicating that the inocula (5 x 103 organisms/foot pad) prepared from the skin biopsies of all 10 patients contained sufficient proportions of viable M. leprae. Although the MEDs of either OFLO or SPFX were different among the 10 strains, the differences were rather small, no more than one dilution of log2. Taking into account the results of all 10 strains, one may conclude that the MED of OFLO is 25 mg/ kg 6 times weekly by gavage or 500 mg/kg/ daily (0.25% w/w) by incorporation into the mouse diet (Table 1). The MED of SPFX is 6.25 mg/kg 6 times weekly by gavage or 100 mg/kg/daily (0.05% w/w) by incorporation into the mouse diet (Table 2).
DISCUSSION
With respect to the MEDs of OFLO or SPFX administered by gavage, the published data suggest that the MED of OFLO may be <50 mg/kg daily (5), and that of SPFX probably between 5 and 10 mg/kg daily (4). However, these results were obtained from experiments with a single strain of M. leprae (4,5). To date, the MEDs of OFLO or SPFX measured by incorporating the drug into the mouse diet are not available. We only know that OFLO in a concentration of 0.05% in the mouse diet, which corresponded to 100 mg/kg/daily, could not inhibit the multiplication of M. leprae in mice (Grosset, unpublished data). In addition, the MED of any antileprosy drug determined by the two methods of drug administration had never been compared in the same experiment.
The results of the current experiment demonstrate that when the drugs were given by gavage 6 times weekly, the MEDs, or the criteria for the diagnosis of resistant M. leprae, of OFLO and SPFX were, respectively, 25 mg/kg and 6.25 mg/kg; these figures are in agreement with the previously estimated values (4,5). However, the MEDs were very significantly higher when the drugs were incorporated into the daily mouse diet: 500 mg/kg/daily for OFLO and 100 mg/kg/daily for SPFX. In either way of drug administration, the anti- M. leprae activity, in terms of the MED, of SPFX was 4-5 times greater than that of OFLO.
The fact that the MEDs of OFLO/SPFX by gavage were 20 times lower than those measured by incorporating the drug into the mouse diet were not totally unexpected, because it has been demonstrated that when OFLO was administered in the mouse diet at a dosage of 800 mg/kg/day, the serum concentrations were maintained at more or less the same level but significantly lower than the peak concentration in mice administered 50 mg/kg of OFLO by gavage.(14)
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
REFERENCES
1. Chan, G. P., Garcia-Ignacio, B. Y., Chavez, V. E., Livelo, J. B., Jimenez, C. L., Parrilla, M. L. R. and Franzblau, S. G. Clinical trial of sparfloxacin for lepromatous leprosy. Antimicrob. Agents Chemother. 38(1994)61-65.
2. Franzblau, S. G. and While, K. E. Comparative in vitro activities of 20 fluoroquinolones against Mycobacterium leprae. Antimicrob. Agents Chemother. 34(1990)229-231.
3. Franzblau, S. G., Parrilla, L. R. and Chan, G. P. Sparfloxacin is more bactericidal than of loxacin against Mycobacterium leprae in mice. Int. J. Lepr. 61(1993)66-69.
4. Gidoh, M. and Tsutsumi, S. Activity of Sparfloxacin against Mycobacterium leprae inoculated into foot pads of nude mice. Lepr. Rev. 63(1992)108-116.
5. Grosset, J. H., Guelpa-Lauras, C. C, Perani, E. G. and Beoletto, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
6. Grosset, J. H., Ji, B., Guelpa-Lauras, C. C, Perani, E. G. and N'Deli, L. N. Clinical trial of Pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281-295.
7. Ji, B. and Grosset, J. H. Recent advances in the chemotherapy of leprosy. Lepr. Rev. 61(1990)313-329.
8. Ji, B., Matsuo, Y. and Colston, M. J. Screening of drugs for activity against Mycobacterium leprae. Int. J. Lepr. 55(1987)836-842.
9. Ji, B., Perani, E. G., Petinon, C. and Grosset, J. H. Bactericidal activities of single and multiple doses of various combinations of new antileprosy drugs and/or rifampin against M. leprae in mice. Int. J. Lepr. 60(1990)556-561.
10. Ji, B., Perani, E. G., Petinon, C, N'Deli, L. N. and Grosset, J. H. Clinical trial of ofloxacin alone and in combination with dapsone plus clofazimine for the treatment of lepromatous leprosy. Antimicrob. Agents Chemother. 38(1994)662-667.
11. Ji, B., Truffot-Pernol, C. and Grosset, J. In vitro and in vivo activities of Sparfloxacin (AT4140) against M. tuberculosis. Tubercle 72 (1991)181-186.
12. Lalande, V., Truffot-Pernot, C, Paccaly-Moulin, A., Grosset, J. and Ji, B. Powerful bactericidal activity of Sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob. Agents Chemother. 37(1993)407-413.
13. Shepard, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 112(1960)445-454.
14. Truffot-Pernot, C, Ji, B. and Grosset, J. Activities of Pefloxacin and ofloxacin against mycobacteria: in vitro and mouse experiments. Tubercle 72(1991)57-64.
1. D.Sc; Institut Marchoux, Bamako, Mali.
2. M.D., D.T.M., M.Sc; Institut Marchoux, Bamako, Mali.
3. M.D.; Institut Marchoux, Bamako, Mali.
4. M.D; Faculté de Médecine Pitie-Salpctricre, Paris, France.
5. M.D.; Faculté de Médecine Pitie-Salpctricre, Paris, France.
Received for publication on 16 October 1995;
Accepted for publication in revised form on 19 December 1995.