- Volume 64 , Number 2
- Page: 146–51
Infection of distal peripheral nerves by M. leprae in infected armadillos; an experimental model of nerve involvement in leprosy
ABSTRACT
Mechanisms of localization of Mycobacterium leprae to the peripheral nerves and of subsequent nerve injury are not understood. No experimental animal model has been available for use in examining the pathogenesis of M. leprae -induced nerve injury. A detailed dissection was, therefore, done of the major peripheral nerves in the extremities of six M. leprae -inoculated armadillos, three of which had developed characteristic disseminated infection.All of the animals with disseminated infection had extensive involvement of the peripheral nerves, increasing in intensity as the nerve was followed distally. No M. leprae were found in the animals without disseminated infection. The degree of infection was greater in cpineural tissues than in the intraneural compartment (i.e., Schwann cells) at all levels. The infection of nerves by M. leprae was associated with focal interstitial, mononuclear cell infiltration of involved nerves.
These results suggest that: 1) armadillos offer a model for the study of neural involvement in leprosy, since the pattern of neural distribution in susceptible armadillos is comparable to the pattern of nerve involvement in man; 2) early localization of M. leprae may be to the cpineural tissues, including lymphatic and vascular structures and extracellular matrix; 3) Schwann cell involvement may be a late event; and 4) mechanisms involving the endothelium of epineural and perineural tissues may be important in the selective localization of M. leprae to peripheral nerves.
RÉSUMÉ
Les mécanismes de localisation de Mycobacterium leprae au niveau des nerfs périphériques et les lésions nerveuses qui s'ensuivent ne sont pas élucidés. Aucun modèle animal expérimental n'a été disponible pour examiner la pathogénèse des lésions nerveuses induites par M. leprae. On a donc fait une dissection minutieuse des principaux nerfs périphériques des extrémités de six tatous à qui on avait injecté de M. leprae et parmi lesquels trois avaient développé une infection disséminée caractéristique.Tous les animaux présentant une infection disséminée avaient une atteinte étendue des nerfs périphériques, augmentant en intensité à mesure qu'on allait vers l'extrémité distale. Aucun M. leprae n'a été trouvé chez les animaux qui n'avaient pas d'infection disséminée. Le degré de l'infection était plus grand dans les tissus epineuraux que dans le compartiment intraneural (les cellules de Schwann) à tous les niveaux. L'infection des nerfs par M. leprae était associée à une infiltration focale, interstitielle, à cellules mononucléaires, des nerfs impliqués.
Ces résultats suggèrent que; 1) les tatous offrent un modèle pour l'étude de l'envahissement nerveux dans la lèpre, puisque le mode de distribution nerveuse chez les tatous susceptibles est comparable au mode d'envahissement nerveux chez l'homme; 2) la localisation précoce de M. leprae pourrait être au niveau des tissus epineuraux, y compris les structures lymphatiques et vasculaires et la matrice extracellulaire; 3) L'envahissement des cellules de Schwann pourrait survenir tardivement; et 4) les mécanismes impliquant l'cndothelium des tissus epineuraux et perineuraux pourraient être importants dans la localisation sélective de M. leprae au niveau des nerfs périphériques.
RESUMEN
Los mecanismos de localización de Mycobacterium leprae en los nervios periféricos y del daño nervioso resultante, son aspectos que no están bien entendidos. No ha habido un modelo animal realmente adecuado para el estudio de la patogénesis de daño a nervios causado por M. leprae. Por esto, en este estudio se hizo una disección detallada de los principales nervios periféricos en las extremidades de 6 armadillos inoculados con M. leprae, tres de los cuales habían desarrollando una característica infección diseminada.Todos los animales con infección diseminada tuvieron una extensa afección de los nervios periféricos que aumentó en intensidad conforme se siguió el transcurso distal del nervio. No se encontraron M. leprae en los animales sin infección diseminada. El grado de infección fue siempre mayor en los tejidos epineurales que en el compartimento intraneural (p. cj. las células de Schwann). La infección de los nervios por M. leprae estuvo asociada con infiltración mononuclear focal e intersticial de los nervios afectados.
Estos resultados sugieren que (1) los armadillos ofrecen un modelo para el estudio de la afección neural en la lepra puesto que el patrón de distribución neural en los armadillos susceptibles es comparable con el patrón de los nervios afectados en el humano; (2) la localización temprana de M. leprae puede ocurrir en el tejido epineural, incluyendo las estructuras linfáticas y vasculares así como la matriz extracelular; (3) la afección de las células de Schwann puede ser un evento más tardío; y (4) los mecanismos que involucran al endotelio de los tejidos epineurales y perineurales pueden ser importantes para la localización selectiva de M. leprae en los nervios periféricos.
Infection of peripheral nerves by Mycobacterium leprae is one of the unique hallmarks of leprosy. Nerve involvement in human leprosy lesions has been studied extensively at both light- and electron-microscopic levels (4,6,7,15,16), but the mechanisms responsible for the unique localization of M. leprae to nerves, and subsequent nerve injury, are not understood.
Although some association of M. leprae with nerves, particularly cutaneous nerves, has been observed in each of the animal models of leprosy (3,10,17,18) none of the nonprimate models has been found to exhibit involvement of major peripheral nerves comparable to that seen in man. In addition, several attempts have been made to induce infection and/or injury to nerves in nonprimate species by direct inoculation of M. leprae into major nerve trunks (5,14) , but no natural or physiologic model of substantial peripheral nerve infection by M. leprae has been described in nonprimates.
The nine-banded armadillo (Dasypus novemcinctus) has been widely used in the production of large quantities of M. leprae since the susceptibility of this animal to M. leprae was first described (9), and the involvement of cutaneous nerves and of the sciatic nerve have been described (1,11,13,18). However, the full extent and pattern of neural involvement in infected armadillos has not been previously reported. This study presents evidence that infected armadillos develop extensive, focal involvement of peripheral nerves which is greater distally and declines proximally, similar to the pattern of nerve involvement in man.
MATERIALS AND METHODS
Six armadillos were examined at necropsy; all had been captured in Florida and observed under quarantine for at least 45 days in captivity prior to inoculation with M. leprae. All of these armadillos were inoculated with M. leprae intravenously: four received 3-4 x 108 armadillo-derived M. leprae, and two received 1.3 x 109 nude mouse-derived M. leprae (strain Thai 53) (The Table). All animals were maintained in closed quarters and monitored for development of systemic infection. Three armadillos developed disseminated infection and were sacrificed to obtain M. leprae; the other three (which did not develop a systemic infection) were sacrificed after prolonged observation.
A full-length dissection of peripheral nerves from one or more extremities was performed on each animal after removal of the viscera. Each nerve was followed to its ramifications in the skin and/or soft tissue, and nerves with their closely associated connective tissues were divided into serial 1 -cm blocks, fixed in 10% neutral buffered formalin, and embedded in paraffin or epoxy resin. The extent of inflammation and the number of acid-fast bacilli in Fite-stained longitudinal sections were determined separately, on a scale of 0-6 + , in the intraneural compartment and in the epineural tissues.
RESULTS
All three of the animals with disseminated M. leprae infection showed extensive nerve involvement, but no M. leprae were found in the peripheral nerves of the three animals which did not develop a disseminated infection (The Table). One animal (N1176) had an increase in cellularity (scattered mononuclear cells not forming granulomas) in some nerves in a lower extremity. No M. leprae were present, and the cause of the increase in cellularity was not evident but it appeared to be nonspecific. In the nerves infected with M. leprae, foci of infection and the number of bacilli per focus were greatest distally and became progressively less intense proximally (Fig. 1). Distally, both deep and superficial nerves were involved.
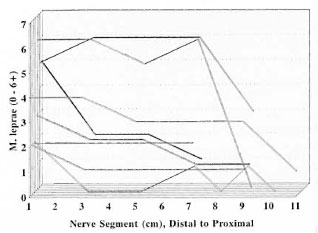
Fig. 1. Distribution of M. leprae along armadillo peripheral nerves. Fite-stained longitudinal sections of 1-cm segments of peripheral nerves were examined for the presence of acid-fast bacilli, and the number of organisms present was recorded on a scale of 0-6 + . Each line represents the findings in an individual nerve plotted from the most distal segment to the most proximal. All involved nerves showed higher numbers of M. leprae distally, decreasing proximally.
The characteristic infection of Schwann cells was seen in all involved nerves (Fig. 2). Perineural and epineural infection and cell proliferation also were seen in numerous foci in heavily infected nerves (Fig. 3). Not uncommonly, bacilli were seen in epineural sites but not in the adjacent intraneural compartment. However, when bacilli were found within nerves, they were nearly always also found in the adjacent epincurium, where they were also more numerous. When the bacterial loads in the intrancural and cpincural tissues were compared, epineurai involvement was always as great as or (usually) greater than intraneural involvement at all levels. Epineurai lymphatics, small blood vessels, and the extracellular matrix all appeared to harbor bacilli-laden macrophages.
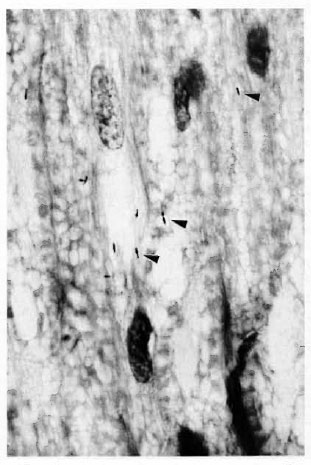
Fig. 2. M. leprae (arrowheads) are seen withinSchwann cells of peripheral nerve in an armadillo withdisseminated infection (Fite stain, 4-μm section, original magnification x800).
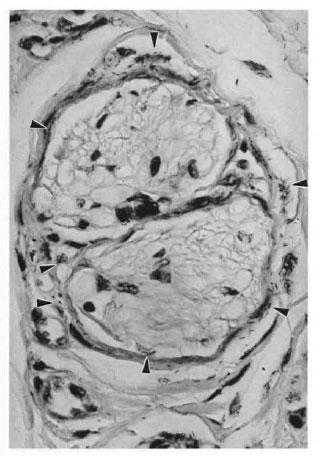
Fig. 3. In armadillo peripheral nerves, M. lepraeare much more abundant in epineural structures (arrowheads) than in the endoneural compartment (Fitestain, 3-μm section, original magnification x177).
The infection elicited a focal chronic inflammatory infiltrate in affected nerves (Fig. 4) comparable to human leprous neuritis.
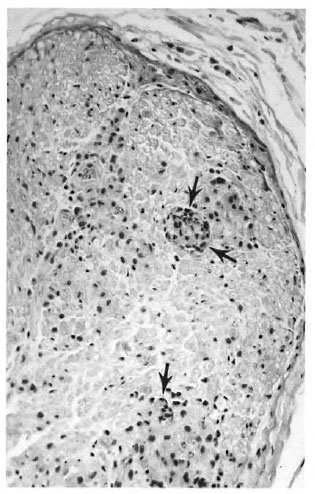
Fig 4. M. leprae infection of armaunio peripneral nerves is accompanied by a multifocal influx of in-flammatory cells into the endoneural compartment (arrows), as in this example from a carpal branch of theulnar nerve near the wrist (hematoxylin and eosin stain,original magnification x69). This results in the histopathologic appearance of neuropathy comparable tothe characteristic M. leprae neuropathy in man.
DISCUSSION
Full-length dissections to examine distal nerve trunks in armadillos have not been done previously and, therefore, the extent of peripheral nerve involvement after inoculation and dissemination of M. leprae has not been appreciated. Sciatic nerve involvement was observed in this study but was not extensive, consistent with previous reports (8,11).
The pattern of nerve involvement in M. leprae- infected armadillos appears to be similar to that seen in man, particularly the increasing degree of involvement as the nerves are followed distally in the extremities. Since this pattern is unique among infectious diseases, its basis is of significance with respect to pathogenesis and may be examined systematically in an animal model. Functional correlates of the bacterial load in these nerves in armadillos are not yet known; paralysis is not seen in infected armadillos, and neither paresis nor sensory deficiencies can be reliably tested in these animals. Duration of infection may be an important factor in the extent and distribution of nerve involvement, since the heaviest infection of nerves was observed in the animal with the longest duration of infection, but the number of animals studied thus far does not permit statistical analysis of the possible significance of this observation.
Involvement of the epineural supporting cells has been noted in nearly all major studies of nerves in leprosy, including human biopsy studies and experimental leprosy in armadillos, but has generally been considered secondary and/or nonspecific. One reason for this is that M. leprae are often more numerous within dermal nerves than in the epineurium, although this is not consistently the case in lepromatous and borderline lepromatous lesions. Factors responsible for this difference between human lesions and the armadillo model may include local tissue differences between dermal nerves and larger nerve trunks, as well as differences in immunologic capabilities and bacterial load in tuberculoid versus lepromatous disease, and the duration of infection before biopsy in these different lesions.
In the present study epineural infection was striking in comparison to the intraneural (Schwann cell) involvement at each level. This is consistent with a pattern of infection in which bacilli are initially arrested in the epineural tissues and subsequently gain entry into the Schwann cells. Such a pathogenetic model predicts that the factors responsible for the localization of M. leprae to nerves may be due to interactions of M. leprae with molecules of the extracellular matrix or with the endothelium of blood vessels or lymphatics found in the epineurium. These events could involve either freely circulating M. leprae or macrophages parasitized with these organisms.
This model also would predict that the involvement of Schwann cells is not necessarily specific. The well-documented phagocytic capability of Schwann cells (2,12) suggests that they would ingest any M. leprae which are able to penetrate the anatomic and physiologic barriers of the perineurium. How M. leprae penetrate these barriers is not explained by the observations presented here, but if large numbers of these bacilli are localized for long periods of time in the epineurium, this would offer a greatly increased probability of bacilli passing through the vasa nervorum which are in direct contact with the intraneural compartment.
The initial observations in this experimental model offer new possibilities in understanding the pathogenesis of nerve injury in leprosy, viewing this as a dynamic succession of adhesion, immunologic, and inflammatory processes occurring at different points during the course of infection. Since bacilli in epineural locations are likely to be more accessible to immunologic effector mechanisms than those within the nerve, proliferation of organisms may be reduced or halted in these sites, while organisms which have reached the endoneural compartment and have been ingested by Schwann cells may be relatively protected. The balance of these events would depend in part on the duration of the infection which, in turn, is partially a function of the patient's immunologic capability toward M. leprae. In this hypothetical model, a point would be reached, at any given site in dermal nerves and nerve trunks, at which bacilli surviving within Schwann cells might prolong the neurologic manifestations of the infection, even after most bacilli in the epineurium and skin have been eliminated.
Armadillos appear to offer an experimental model for nerve involvement and injury in Hansen's disease. The model could prove useful in addressing mechanisms of localization of M. leprae to nerve, mechanisms of nerve injury, and effects of antimicrobial therapy. Since it represents localization to nerves by a natural, physiologic process, this model could be useful even if the extensive epineural and intraneural involvement by M. leprae observed in these armadillos were only a manifestation of the late stages in dissemination of the infection. Whether this is true or not and the pattern of neural involvement which may be seen early in the course of infection are matters now under investigation.
Acknowledgment. We are grateful to J. Allen, A. Deming, and G. McCormick for their technical assistance and to Mrs. Penne Cason for her assistance in preparing the manuscript.
REFERENCES
1. Balentine, J.D., Chang, S.C. and Issar, S.L. Infection of armadillos with AI. leprae, ultrastructural study of peripheral nerve. Arch. Pathol. Lab. Medicine 100(1975)175-181.
2. Band, H., Battacharya, A. and Talwar, G.P. Mechanism of phagocytosis by Schwann cells. J. Neurol. Sci. 75(1986)113-119.
3. Chehl, S., Job, C.K. and Hastings, R.C. Transmission of leprosy in nude mice. Am. J. Trop. Med. Hyg. 34(1985)1161-1166.
4. Dastur, D.K. Pathology and pathogenesis of predilectivc sites of nerve damage in leprous neuritis. Neurosurg. Rev. 6(1983)139-152.
5. de Blaquiere, G.E., Santamaria, L., Curtis, J., Terenghi, G., Polak, J.M. and Turk, J.L. A morphological and functional assessment of M. leprae -induced nerve damage in a guinea pig model of leprous neuritis. Neuropathol. Appl. Neurobiol. 20(1994)261-271.
6. Job, C.K. Nerve damage in leprosy. Int. J. Lepr. 57(1989)532-539.
7. Job, C.K. M. leprae in nerve lesions in lepromatous leprosy. Arch. Pathol. 89(1970)195-207.
8. Job, C.K., Sanchez, R. and Hastings, R.C. Manifestations of experimental leprosy in the armadillo. Am. J. Trop. Med. Hyg. 34(1985)151-161.
9. Kirchheimer, W.F. and Storrs, E.E. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of Iepromatoid leprosy in an experimentally-infected armadillo. Int. J. Lepr. 39(1971)693-702.
10. Kirschheimer. W.F., Storrs, E.E. and Binford, CH. Attempts to establish the armadillo (Dasypus novemcinctUS) as a model for leprosy. 2. Histopathologic and bactériologie post-mortem findings in lepromatous leprosy in the armadillo. Int. J. Lepr. 40(1972)229-242.
11. Liu, T.C., Ji, Z.M. and Skinsnes, O.K. Light-and electron-microscopic study of M. leprae-infected in Armadillo Leprosy 151 armadillo nerves. Int. J. Lepr. 57(1989)65-72.
12. Lumsden, C.E. Leprosy and the Schwann cell. In: Leprosy in Theory and Practice. Cochrane, R.G. and Davcy, T.F., cds. Bristol: John Wright and Sons, 1964, pp. 221-250.
13. Mehta, L. and Antia, N.H. Ultrastructure of sciatic nerve of armadillo infected with M. leprae. Indian J. Lepr. 56(1984)540-554.
14. Mshana, R.N., Humber, D.P., Harboe, M. and Belehu, A. Nerve damage following intraneural injection of M. leprae into rabbits pre-sensitized to mycobacteria. Clin. Exp. Immunol. 52(1983)441-448.
15. Sabin, T.R., Swift, T.R. and Jacobson, R.R. Leprosy. In: Peripheral Neuropathy. 3rdedn. Dyck, P.J. and Thomas, P.K., eds. Philadelphia: W.B. Saunders, 1993, pp. 1354-1379.
16. Shetty, V.P., Antia, N.H. and Jacobs, J.M. The pathology of early leprous neuropathy. J. Neurol. Sci. 88(1988)115-131.
17. Wolf, R.H., Gormus, B.J., Martin, L.N., Baskin, G.B., Walsh, G.P., Meyers, W.M. and Binford, C.H. Experimental leprosy in three species of monkeys. Science 227 (1985)529-531.
18. Yoshizumi, M.O., Krischheimer, W.F. and Asbury, A.K. A light and electron microscopic study of peripheral nerve in an armadillo with disseminated leprosy. Int. J. Lepr. 42(1974)251-259.
1. M.D., Ph.D., Department of Research Pathology.
2. D.V.M.; Department of Laboratory Animal Care.
3. Ph.D.; Department of Microbiology, Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P. O. Box 25072, Baton Rouge, LA 70894, U.S.A.
Received for publication on 2 October 1995;
Accepted for publication in revised form on 9 January 1996.