- Volume 64 , Number 2
- Page: 152–8
Evolution of lymphocyte populations in armadillos (Dasypus novemcinctus) inoculated with M. leprae
ABSTRACT
In human leprosy patients there are changes in the percentages of T and B lymphocytes in peripheral blood, and there is a correlation with the clinical characteristics or manifestations of the disease. These phenomena still require clarification regarding the triggering mechanism involved that may lead to one or the other clinical entities. Much has yet to be learned about the intricacies of whether the changes in subpopulations of T and B lymphocytes are a causative factor or an effect attributable to the microorganism itself.The armadillo is an excellent animal model to study how Mycobacterium leprae spread, turning into an established infection. The application of modifications in percentages of the subpopulations of B and T lymphocytes in armadillos may well lead to extrapolation of the results obtained in this animal model in an attempt to be able to manipulate the course of the disease in humans.
The purpose of the study was to evaluate changes in the percentages of rosette-forming and sIgM+ mononuclear cells during a full year in groups of armadillos: five randomly chosen animals formed the control group and 11 armadillos were inoculated with M, leprae obtained f rom a human leproma at the onset of the 12-month period of the study. Of the 11 randomly selected armadillos that were inoculated, only five developed an active and disseminated infection. The percentage of rosette-forming cells did not show statistically significant variations during the first 6 months of the study. However, at months 8 and 12 a significant increment in this parameter was observed (p < 0.05) in the animals with active infection. In regard to the variations in the numbers of slgM+ cells, significant changes occurred in the armadillos with active infection at month 2. However, results returned to normal and no changes were seen at later times. No significant changes occurred in the group of animals inoculated but not developing active infection compared with the other groups.
The results are considered sufficiently interesting to encourage further study on the cell-mediated immune system of the armadillo and the changes that occur during the development and dissemination of an inoculated infection with M. leprae. Since this mammal is of great value as an effective animal model in the experimental research of M. leprae, there is an urgent need to obtain, as quickly as possible, a thorough understanding of the cellular branch of its immune system and, thereby, be in a position to extrapolate immune modulation to benefit human leprosy patients.
RÉSUMÉ
Des modifications ont lieu, chez les malades de la lèpre, dans les pourcentages de lymphocytes T et B dans le sang périphérique, et cela est correlé avec les caractéristiques cliniques ou les manifestations de la maladie. Ces phénomènes doivent encore être éclaircis en ce qui concerne le mécanisme déclencheur impliqué qui peut conduire à l'une ou l'autre des entités cliniques. On doit encore beaucoup apprendre sur la question de savoir si les modifications observées dans les sous-populations de lymphocytes T et B sont un facteur causal ou un effet attribuable au microorganisme luimême.Le tatou est un excellent modèle animal pour étudier comment Mycobacterium leprae se propage, pour aboutir à une infection bien établie. L'application des modifications dans les pourcentages des sous-populations de lymphocytes B et T chez le tatou pourrait conduire à une extrapolation des résultats obtenus dans ce modèle animal dans la tentative de manipuler le décours de la maladie chez l'homme.
Le but de l'étude était d'évaluer les modifications dans les pourcentages de cellules mononucléaires formant des rosettes et slgM + durant une année complète dans un groupe de tatous: cinq animaux choisis au hasard ont formé le groupe-témoin et 11 animaux ont été inoculés avec du M. leprae obtenu d'un léprome humain au début de la période de 12 mois qu'a duré l'étude. Des 11 tatous sélectionnés au hasard qui ont été inoculés, 5 seulement ont développé une infection active et disséminée. Le pourcentage de cellules formant rosettes n'a pas montré des variations statistiquement significatives durant les 6 premiers mois de l'étude. Cependant, aux mois 8 et 12, une augmentation significative de ce paramètre a été observée (p < 0.05) chez les animaux avec une infection active. Vu les variations dans le nombre de cellules slgM +, des modifications significatives sont survenues au mois 2 chez les tatous avec une infection active. Cependant, les résultats sont revenus à la normale et aucun changement n'a été observé plus tard. Aucun changement significatif n'est survenu dans le groupe des animaux inoculés mais qui n'ont pas développé d'infection active, par comparaison aux autres groupes.
Les résultats sont considérés suffisamment intéressants pour encourager des études ultérieures sur le système immunitaire à médiation cellulaire du tatou et les modifications qui surviennent durant le développement et la dissémination d'une infection inoculée avec M. leprae. Puisque ce mammifère est de grande valeur en tant que modèle animal dans la recherche expérimentale sur la lèpre, il y a un besoin urgent d'obtenir, aussi vite que possible, une compréhension profonde de la branche cellulaire de son système immunitaire et, par là, être en position d'extrapoler la modulation immunitaire au bébélice des malades de la lèpre.
RESUMEN
En los pacientes con lepra ocurren cambios en sus porcentajes de linfocitos T y B circulantes que correlacionan con las características clínicas o con las manifestaciones de la enfermedad. Los mecanismos que conducen a las diferentes formas de la lepra aún quedan por ser aclarados y todavía hay mucho que aprender para comprender si los cambios en las subpoblaciones de linfocitos T y B son causa o el resultado de la enfermedad. El armadillo es un excelente modelo animal para estudiar cómo se disemina Mycobacterium leprae y cómo se establece para causar enfermedad. El estudio sobre los cambios en las subpoblaciones de las células T y B en el armadillo podría ayudar a entender la inmunopatología de la enfermedad en el humano.El propósito de este estudio fue el de evaluar los cambios en los porcentajes de las células mononucleares formadoras de rosetas y de las células sIgM + en armadillos infectados con M. leprae. Cinco armadillos seleccionados al azar sirvieron como grupo control y 11 animales fueron inoculados con M. leprae de origen humano. El estudio se sostuvo durante un año a partir del tiempo de infección. De los once armadillos inoculados sólo 5 desarrollaron una infección activa y diseminada. Los porcentajes de células formadoras de rosetas no mostraron variaciones estadísticamente significantes durante los primeros 6 meses del estudio. Sin embargo, a los 8 y los 12 meses de infección se observó un incremento significante en este parámetro (p < 0.05) en los animales con la infección activa. En cuanto a las variaciones en los números de células sIgM + , se observaron cambios significativos en el mes 2 en los armadillos con infección activa. No obstante, los resultados se normalizaron en los tiempos posteriores del estudio y no se observaron diferencias significativas entre los animales inoculados que desarrollaron la infección activa y aquellos de los otros grupos.
Los resultados alientan el estudio de la inmunidad celular y de los cambios que suceden durante el desarrollo y la diseminación de la enfermedad en los armadillos infectados con M. leprae. Puesto que el armadillo es un mamífero de gran valor para el estudio de la infección experimental con M. leprae, la exploración del sitema inmune celular de este animal podría permitir su modulación y, por extrapolación, la modulación de la respuesta inmune celular en los humanos afectados por la lepra.
Two distinct clinical presentations of Mycobacterium leprae infection in man correlate with changes found in the T and B lymphocytes of leprosy patients (6,9,19) . It is questionable if these alterations in the lymphocyte characteristics are responsible for the variations in the clinical manifestations of tuberculoid leprosy (TL) or lepromatous (LL) leprosy. Recent research has suggested that T lymphocytes from LL patients produce an interleukin pattern in which interleukin-4 (IL-4), IL-5 and IL-10 are identifiable (13,21,22). The preponderance of these interleukin moieties could explain the existence of anergy in the T-cell population and the exacerbation in the humoral responses manifest in this specific type of patients. Still needing explanation is whether the clinical entities are dependent on a predisposition of the infected individual toward one or the other type of lymphocyte response, or whether the microorganism per se is the determining factor. The answer is not clearly established since we still do not understand why T cells from LL patients generate a Th-2 response while patients with TL give rise to a Th-1 interleukin pattern. T-cell supressor activity has been claimed to be responsible for the specific anergy seen among LL patients; CD8 T-cell clones taken from these patients generated a Th-2 interleukin pattern (11). Other factors, different than IL-4 or IL-10 also have been claimed to be responsible for this anergy (7). The role of IL-12 (15,17) and the recognition of nonpeptide antigens from M. leprae presented to T cells by human CD 1 b molecules should be evaluated in both tuberculoid and lepromatous leprosy ( ) to analyze how they contribute to protection or anergy against M. leprae. To explain the process involved in the initial stages of M. leprae infection, an animal model may be quite helpful.
Armadillos with an induced infection with M. leprae (5,16) develop a lepromatous-type infection, and spontaneous lepromatous leprosy in these animals has been described (1,20). There is little information concerning the immune responses related to M. leprae infection in the nine-banded armadillo (Dasypus novemcinctus). The data focus on antibody production (4,18) and, presumably, changes in the cellular branch of immunity were omitted due to a lack of the required reagents.
There are published results on specific lymphocyte markers for identification of these cell populations in armadillos. Rosette formation with sheep red blood cells (SRBC) (2,3,12), Fc receptors (3), C3b receptors (2,3) and the presence of surface Ig (2,3) are reported in the literature. Strangely, no control comparisons between healthy versus inoculated armadillos appears in these publications.
In the present study, particular interest has been given to the analysis of cellular changes in armadillos inoculated with M. leprae. Prior to this, a study on two lymphocyte markers for lymphocytes from armadillos was performed: formation of rosettes with SRBC and the identification of lymphocyte surface IgM (12). Comparing these cell populations and the modifications that occur was begun many months before the initiation of this study, and provided a firm basis for the acquisition of internal controls of the parameters under investigation in these same armadillos.
MATERIALS AND METHODS
Armadillos. Armadillos were captured in the highlands of Michoacan, Mexico, and were kept in captivity as previously described (8). Eleven of these animals, randomly chosen, were inoculated with 1 x 108 M. leprae obtained from human lepromas. Five additional armadillos, also randomly chosen, were included as the control group.
Collection of blood samples. After fasting overnight, the armadillos were anesthetized with Ketalar (a ketamine base) 35 mg/kg (administered intramuscularly), and immediately an 8-ml sample of blood was withdrawn from each animal by cardiac puncture. The blood was mixed with 5 ml of Alsever's solution, and the mononuclear cells were separated as described subsequently.
Determination of surface IgM bearing lymphocytes and rosette-forming cells. The mononuclear cells were purified from peripheral blood using Histopaque 1.077 (Sigma) and then stained with the FITC conjugated rabbit IgG anti-armadillo IgM as we described previously (12), or evaluated for their capacity to form rosettes with SRBC (2,3,12) Briefly, armadillo lymphocytes (1 x 106) were mixed with a suspension of SRBC (1 x 108). The mixture was incubated for 15 min at 37ºC, centrifuged for 3 min at 400 x g, and then incubated overnight at 4ºC. Enumeration of rosette-forming cells was done by counting the lymphocytes with three or more SRBC attached to their surface (12).
RESULTS
In a previous study (l2), the percentage of rosette-forming cells and surface IgM-bearing lymphocytes of noninoculated, healthy armadillos was undertaken, and the investigation was extended for the inclusion of the results for the present investigation. Twenty-two healthy armadillos permitted the establishment of investigative results that provided the base value of 14.2 ± 8.1% rosette-forming cells, and 15.6 ± 5.5% (mean ± S.D.) surface-bearing-IgM lymphocytes. These values are utilized for derivation of the kinetics involved in establishing the results of the present study.
There was a degree of blindness built into the present investigation since the person evaluating the repercussion on the inoculated animals of the M. leprae infection was completely independent of those involved in quantifying lymphocytes. At the completion of the study, the files were opened to all involved in this line of research.
The analysis involving all groups participating in this research was characterized as follows: Five of the 11 armadillos inoculated with M. leprae developed an obvious infection easily detectable on sight within 9 months of the initial inoculation. Smears of nasal secretion were positive for acid-fact bacilli (AFB) as well as lymph obtained from scarification of the ears of the animals. Autopsies of the infected armadillos revealed dissemination of lepromas throughout different parts of the body, as well as marked hepato-splenomegaly. This group is referred to as "inoculated with infection" (IWI). In 6 of the 11 inoculated armadillos no evidence of infection was present. The nasal smears and lymph obtained from the ears were negative for the presence of AFB. This group was designated as "inoculated without infection" (Iw/ol). The initial control group made up of five randomly picked armadillos was designated as "not inoculated" (NI).
Rosette formation was quite variable during the months of the investigation (Fig. 1). No clear correlation could be established between armadillos inoculated with M. leprae and the values of the lymphocyte population observed by this method. Notwithstanding, the animals that did develop infection were found to have the greatest variations in the number of rosette-forming mononuclear cells. In addition it was possible to detect significant increases in the number of rosette-forming cells (p < 0.05) at months 8 and 12 after inoculation in comparison with the internal control values obtained from the very same animals before administration of the M. leprae. The increases at months 8 and 12 were equally statistically significant when compared to the other established groups indicated above. That is to say, a p < 0.05 was found to be present in comparison with the NI versus the IWI and the Iw/ol groups. The inoculated animals that did not develop infection had variations in the numbers of rosetteforming cells similar to those observed in the control or noninoculated group.
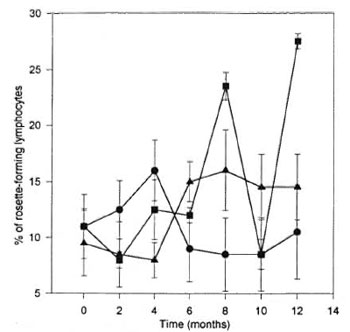
Fig. 1. Percentage of rosette-forming lymphocytes from armadillos inoculated with M. leprae. Eleven armadillos (Dasypus novemcinctus) were inoculated with M. leprae; 5 animals developed a clear infection ( ); 6 animals were free of infection at the end of the study (
); 6 animals were free of infection at the end of the study ( ); 5 noninoculated animals were used as controls (
); 5 noninoculated animals were used as controls ( ). All armadillos were bled every 2 months and the percentage of rosette-forming cells was evaluated. Each point represents the mean ± S.E.M. of each group of animals.
). All armadillos were bled every 2 months and the percentage of rosette-forming cells was evaluated. Each point represents the mean ± S.E.M. of each group of animals.
Variations of surface IgM-bearing lymphocytes are shown in Figure 2. The variability is also apparent. An increase did occur in the IWI group which was statistically significant (p < 0.05) in comparison with its own internal control value established before the inoculation, but it was not statistically significant when compared with the other groups. While the increase in the IWI was not significant in comparison with the control group, a similar tendency to increase did not appear in the controls.
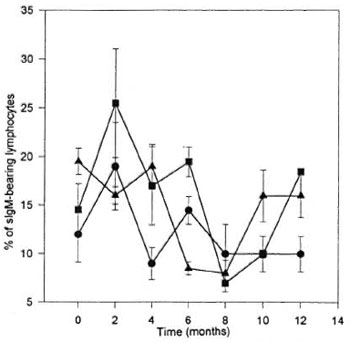
Fig. 2. Percentage of surface-IgM-bearing lymphocytes from armadillos inoculated with M. leprae. Eleven armadillos (Dasypus novemcinctus) were inoculated with M. leprae; 5 animals developed a clear infection ( ); 6 animals were free of infection at the end of the study (
); 6 animals were free of infection at the end of the study ( ); 5 noninoculated animals were used as controls (
); 5 noninoculated animals were used as controls ( ). All armadillos were bled every 2 months and the percentage of surface-IgM-bearing lymphocytes was evaluated. Each point represents the mean ± S.E.M. of each group of animals.
). All armadillos were bled every 2 months and the percentage of surface-IgM-bearing lymphocytes was evaluated. Each point represents the mean ± S.E.M. of each group of animals.
DISCUSSION
The clinical and histopathological manifestations of leprosy in humans present in the form of two distinct entities. The disparities in the manifestations have been attributed to differences in the response of the immune system. Evidence has been accumulated which indicates that the differences in the clinical and histological manifestations in patients with LL may be attributed to a decrease in the response of the cellmediated branch of the immune system. This is implied by the finding of a reduction in the percentage of T-cell lymphocytes in peripheral blood of those patients presenting with LL and a concomitant decrease in cell-mediated immunity against the invading and highly disseminated microorganism( 6,9,19). The T lymphocyte subsets from peripheral blood of these patients have been quantified, and show an increment in CD8 lymphocytes, giving rise to the observed decrease in the CD4/CD8 cell ratio (6,9,19).
There appears to be no available report on markers defining T- and B-cell populations in armadillos. Nevertheless, there are some publications which mention the existence of some lymphocyte cell receptors in these animals (i.e., SRBC receptors and 2,3,12) the surface IgM2- The existence of a means to evaluate lymphocyte subpopulations in armadillos encouraged the design of the present study in which variations in healthy as well as M. leprae inoculated animals took place.
Balina, et al. (2) have quantified rosette formation in lymphocytes in Dasypus hybridus , and reported values of approximately 8%. Escobar, et al. (3) published their findings in D. novemcinctus of <49% of rosette formation in the total lymphocyte population in the same species of armadillos utilized in our study. Figure 1 shows our present overall findings in all groups of armadillos in which rosette formation was performed. In the five randomly selected healthy armadillos chosen as controls, the percentage of rosette-forming lymphocytes was variable with a mean ± S.D. of 6.6% (Fig. 1). Marked variations were not apparent in the group of Iw/ol animals while, in contrast, the IWI armadillos showed two statistically significant peaks (p < 0.05) at 8 and 12 months of the study in comparison with the other groups. It is tempting to propose some meaning to the increments observed in the IWI group, albeit no clear-cut association may be established between the presence of this marker and any specific function in itself.
The cells carrying the surface IgM marker may well be part of the B-cell lymphocyte population (12). Balina, et al. (2) reported the presence of this marker in 27% of the mononuclear cell population of peripheral blood from D. hybridus. In our current study the values found in the corresponding peripheral blood samples from D. novemcinctus were much lower, 15.6% ± 5.5%. The variation may reside in the different armadillo strain as well as the higher specificity of the anti-IgM utilized in our evaluation which identifies IgM heavy chains exclusively (12). The discrepancies in the percentage reported by Balina, et al. in a different strain has been overcome by undertaking additional studies in our own research unit employing rabbit IgG against both heavy and light chains of the IgM marker for the detection of subpopulations of peripheral blood mononuclear cells in D. novemcinctus. With this antibody, the percentage of surface immunoglobulin-bearing lymphocytes was found to be in the vicinity of 30%, very similar to the value reported by Balina, et al. in D. hybridus. No correlation was established between the presence of surface IgM-bearing lymphocytes (slgM) and the existence of active infection. A variation with statistical significance (p < 0.05) was discernible in the IWI group 2 months after initiation of the trial which consisted of a rise in the number of slgM in the inoculated animals. However, all values declined back to normal values without any significant variations in this subpopulation of mononuclear cells at any later date.
The observations derived from the evaluation of the control group, and the independence that took place between those in charge of assessing the condition of the animals, contributed to the realization of a blind evaluation by those dealing with the changes in the peripheral lymphocytes. Our belief is that the findings uncovered are unrelated to environmental or nutritional changes since the armadillos were kept in the same quarters and had access to the same food sources. Whatever difference that may be derived from the study of Figure 1 and Figure 2 is considered attributable to the response of the armadillos to the inoculated infection, or its repercussion during the process thwarting its invasiveness, or the development and subsequent dissemination in the five armadillos that developed active infection.
Studies in mice with M. lepraemurium have been conducted. Roch, et al. (10) analyzed the percentages of lymph node lymphocytes 15 days after inoculating mice with M. lepraemurium, and the sIgM+ cells increased and remained elevated during the observation period of the active infection. In that publication, reference to a decrease in Thy-1 + cells was found to be present by the second month.
Correlation of the T- and B-cell changes have been evaluated in patients having active infection due to M. leprae. The modification in cell percentages is more marked in lymphocytes infiltrating the proliferating lesions (6,9,15,9).
While the results so far obtained are not yet comparable to those found in human disease and the results in experimental inoculation in mice, there is reason to believe that further investigation on T- and B-cell markers in armadillos harboring the human M. leprae strain may aid in a better understanding of the development of lepromatous leprosy in this animal model. Ongoing work in our laboratory is indicating that lectins may be useful to identify armadillo lymphocyte subpopulations (Santos-Argumedo L., et al., Int. J. Lepr., in press). The proliferative response of M. leprae- infected armadillos to lectins is also being evaluated, and some preliminary results have shown a decrease in the response to concanavalin A correlating with the progress of the infection (Rivero-Nava L., et ai, manuscript in preparation).
Armadillos are an asset for further research in our understanding of the participation of the cell-mediated branch of the immune system and its changes during the development of infection following inoculation of M. leprae into this animal model.
Acknowledgment. This work was supported by grants from the Consejo Nacional de Ciencia y Tecnología, México D.F., México, and the Dirección de Estudios de Postgrado e Investigación, I.P.N., México. The authors hold fellowships from COFAA, BDA and/or SNI, México. We thank Dr. Cesar Calva-Pellicer for his critical reading of this manuscript.
REFERENCES
1. Amezcua, M. E., Escobar-Gutierrez, A., Storrs, E. E., Dhople, A. M. and Burchfield, H. F. Wild Mexican armadillo with leprosy-like infection. (Letter). Int. J. Lepr. 52(1984)254-255.
2. Baliña, L. M., Cardama, J. E., Gahi, J. C, Valdez, R. P. and Bianchi, O. Research on armadillos in Argentina. In: The Armadillo as an Experimental Model in Biomedical Research. Washington, D.C.: Pan American Health Organization, 1978, pp. 103-106. Sci. Pub. 366.
3. Escobar-Gutierrez, A. and Amezcua, M. E. El armadillo: un nuevo animal de experimentación para el estudio de las zoonosis. Cien. Vet. 3(1981)200-224.
4. Job, C. K., Drain, V., Truman, R., Deming, A. T., Sanchez, R. M. and Hastings R. C. The pathogenesis of leprosy in the nine-banded armadillo and the significance of IgM antibodies to PGL-1. Indian J. Lepr. 64(1992)137-151.
5. Kjrchheimer, W. F. and Storrs, E. E. Attemps to establish the armadillo (Dasypus novemcinctus Linn) as model for study of leprosy. Int. J. Lepr. 39 (1971) 693-702.
6. Mshana, R. N., Haregewoin, A., Harboe, M. and Belehu, A. Thymus dependent lymphocytes in leprosy. I. T lymphocyte subpopulations defined by monoclonal antibodies. Int. J. Lepr. 50(1982)291-296.
7. Mutis, T., Kraakman, E. D., Cornelisse, Y. E., Haanen, J. B. A. G., Spits, H., de Vries, R. R. P. and Ottenhoff, T. H. M. Analysis of cytokine production by Mycobacterium-reacûve T cells. J. Immunol. 150(1993)4641-4651.
8. Quesada-Pascual, F., Rojas-Esimnosa, O., Santos-Argumedo, L. and ESTRADA-PARRA, S. A Mexican armadillo (Dasypus novemcinclus) colony for leprosy research. Int. J. Lepr. 55(1987)716-718.
9. Rea, T. H., Bakke, A. C, Parker, J. W., Modlin, R. L. and Horwitz, D. A. Peripheral blood T lymphocyte subsets in leprosy. Int. J. Lepr. 52 (1984) 311-317.
10. Roch, F. and Bach, M.-A. Strain differences in mouse cellular responses to Mycobacterium lepraemurium and BCG subcutaneous infections. I. Analysis of cell surface phenotype in local granulomas. Clin. Exp. Immunol. 80(1990)332-338.
11. Salgame, P., Abrams, J. S., Clayberger C, Goldstein, H., Convit, J., Modlin, R. L. and Bloom, B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 254(1991)279-282.
12. Santos-Argumedo, L., Guerra-Infante, F., Quesada-Pascual, F. and Estrada-Parra, S. Identification and purification of armadillo (Dasypus novemcinctus) immunoglobulins: preparation of specific antisera to evaluate the immune response in these animals. Int. J. Lepr. 63(1995)56-61.
13. Sieling, P. A., Abrams, J. S., Yamamura, M., Salgame, P., Bloom, B. R.. Rea. T. H. and Modlin, R. L. Immunosupressive roles for IL10 and IL-4 in human infection: in vitro modulation of T cells responses in leprosy. J. Immunol. 150(1993)5501-5510.
14. Sieling, P. A., Chatterjee, D., Porcelli, S. A., Prigozy, T. I., Mazzaccaro, R. J., Soriano, T., Bloom, B. R., Brenner, M. J.. Kronenberg, M., Brennan, P.J. and Modlin, R. L. CD 1-restricted T cell recognition in microbial lipoglycan antigens. Science 269 (1995)227-230.
15. Sieling, P. A. and Modlin, R. L. Cytokine patterns at the site of mycobacterial infection. Immunobiology 191(1994)378-387.
16. Storrs, E. E., Walsh, G. P. and Burchfield, H. F. Development of leprosy in another species of armadillo Dasypus hybridus; genetic and immunologic implications. J. Trop. Med. 78(1975)216-218.
17. Trinchieri, G. Interleukin-12: a pro-inflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13(1995)251-276.
18. Vadiee, A. R., Shannon, E. J., Gillis, T. P., Mshana, R. N. and Hastings, R. C. Armadillo IgG and IgM antibody responses to phenolic glycolipid-I during experimental infection with M. leprae. Int. J. Lepr. 56(1988)422-427.
19. Wallach, D., Cottenot, F. and Bach, M.-A. Imbalances in T cell in lepromatous leprosy. Int. J. Lepr. 50(1982)282-290.
20. Walsh, G. P., Storrs, E. E., Burchfield, H. F., Cottrell, E. H., Vidrine, M. F. and Binford, C. H. Leprosy-like disease occurring naturally in armadillos. J. Reticulocndothel. Soc. 18(1975)347-351.
21. Yamamura, M., Uyemura, K., Deans, R. J., Weinberg, K., Rea, T. H., Bloom, B. R. and Modlin. R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277-279.
22. Yamamura, M., Wang, X. M., Ohmen, J. D., Uyemura, K., Rea, T. H., Bloom, B. R. and Modlin, R. L. J. Cytokine of immunologically mediated tissue damage. Immunology 149(1992)1470-1475.
1. M.Sc.; Department of Immunology, Escuela Nacional de Ciencias Biológicas, I.P.N., Prol. Carpió y Plan de Ayala, Mexico DF 11340, Mexico.
2. M.Sc; Department of Immunology, Escuela Nacional de Ciencias Biológicas, I.P.N., Prol. Carpió y Plan de Ayala, Mexico DF 11340, Mexico.
3. Ph.D., Department of Immunology, Escuela Nacional de Ciencias Biológicas, I.P.N., Prol. Carpió y Plan de Ayala, Mexico DF 11340, Mexico.
4. Ph.D., Department of Cell Biology, Centro de Investigación y Estudios Avanzados, I.P.N., Apartado Postal 14-740, Mexico DF 07000, Mexico.
Reprint requests to Dr. Santos-Argumedo at above address or FAX 52-5-747-7002 or e-mail santos@cell.cinvestav.mx
Received for publication on 7 June 1995;
Accepted for publication in revised form on 5 December 1995.