- Volume 64 , Number 2
- Page: 159–65
Ultrastructure of normal armadillo epidermis
ABSTRACT
In view of the importance of the ninebanded armadillo (Dasypus novemcinctus) in leprosy research, we studied the ultrastructure of the normal epidermis of this species. The three basic cell types of human epidermis were identified in armadillo skin: keratinocytes, melanocytes, and Langerhans' cells. The role of Langerhans' cells in the human cell-mediated immune system and the description of changes in the number and structure of Langerhans' cells in human leprosy make detailed observations of these cells in the armadillo highly relevant. Clear cells with ultrastructural features typical of Langerhans' cells were observed in normal armadillo epidermis in all areas of skin sampled (abdomen, chin, ear, and thigh), but are fewer than in human skin. These baseline findings are valuable for further studies on Langerhans' cells and the cell-mediated immune function in armadillos with naturally acquired or experimental leprosy.RÉSUMÉ
Vu l'importance du tatou à neuf bandes (Dasypus novemcinctus) dans la recherche sur la lèpre, nous avons étudié l'ultrastructurc de l'épiderme normal de cette espèce. Les trois types de cellules de base de l'épiderme humain ont été identifiés dans la peau du tatou: kératinocytes, mélanocytcs et cellules de Langerhans. Le rôle des cellules de Langerhans dans le système immunitaire humain à médiation cellulaire et la description des modifications dans le nombre et la structure des cellules de Langerhans dans la lèpre humaine ont rendu tout à fait appropriées les observations de ces cellules chez le tatou. Des cellules claires avec des caractéristiques ultrastructuralcs typiques des cellules de Langerhans ont été observées dans l'épiderme normal du tatou dans toutes les régions cutanées examinées (abdomen, menton, oreille et cuisse), mais en nombre moindre que dans la peau humaine. Ces observations de départ sont importantes pour des études ultérieures sur le rôle des cellules de Langerhans dans l'immunité à médiation cellulaire chez des tatous avec une lèpre acquise de manière naturelle ou expérimentale.RESUMEN
En vista de la importancia del armadillo de 9 bandas (Dasypus novemcinctus) en las investigaciones sobre la lepra decidimos estudiar la ultraestructura de la epidermis normal en esta especie. En la piel de los armadillos se identificaron los 3 tipos celulares básicos de la epidermis humana: queratinocitos, melanocitos, y células de Langerhans. El papel de las células de Langerhans en la inmunidad celular y la descripción de los cambios en el número y la estructura de estas células en la lepra de los humanos hace que el estudio de estas células en el armadillo sea un aspecto sumamente relevante. Se observaron células con las características ultraestructureales típicas de las células de Langerhans en la epidermis normal del armadillo de todas las áreas investigadas (abdomen, cachete, oreja y pierna) aunque su número fue menor que el correspondiente a la piel humana. Estos hallazgos básales podrían ser de valor cuando se estudie el papel de las células de Langerhans en la piel de los armadillos con lepra natural o experimental.Identification of the nine-banded armadillo as a model for lepromatous leprosy is a landmark in leprosy research (7,16). Histopathologic and immunologic studies of both experimental and naturally acquired leprosy in armadillos (1,6,18) suggest a defect in cell-mediated immunity (CMI) similar to the defect in lepromatous leprosy in humans. Previously, observations on CMI in armadillos have concentrated on peripheral blood lymphocytes and macrophages (12,13). Langerhans' cells have not been described previously in normal or infected armadillos. This report describes the structure of the epidermis in normal armadillos and provides baseline data on the distribution, number and morphology of Langerhans' cells in normal armadillo skin. These cells are believed to be involved in antigen presentation and T-ccll sensitization (5,14,15,18). Langerhans' cells in lesions of leprosy in the skin in humans vary in numbers and morphology (2,3,9,11).
MATERIALS AND METHODS
Punch (4-mm) biopsy specimens of skin were taken under ketaminc anesthesia from four, normal, adult nine-banded armadillos (Dasypus novemcinctus) captured in the state of Louisiana, U.S.A., and housed in the Armed Forces Institute of Pathology, Washington, DC, U.S.A. Leprosy had been excluded by repeated examination of smears of tissue fluid from the skin of the ears for acid-fast bacilli. Anatomical areas sampled are shown in The Table. Specimens were fixed in 2.5%glutaraldyhyde, postfixed with osmium tetroxide, dehydrated with concentrations of graded alcohol and embedded in Spurr's medium. Semithin sections (1.0-μm) were stained with toluidine blue and studied by light microscopy. Ultrathin sections were cut and stained with uranyl acetate and lead citrate, and examined with a Zeiss 109 electron microscope.
RESULTS
The general structure of the epidermis was similar to that of human skin (Fig. 1). The epidermis varied from live to seven cell layers thick. The epidermal basement membrane, basal cell layer, squamous layer, granular layer, and stratum corneum layer were identified.
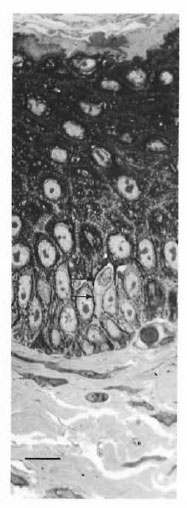
Fig. 1. Semithin (1-μm) plastic embedded section of abdominal skin of armadillo. Note clear cell with indented nucleus (arrows) (toluidine blue stain, bar = 10 μm).
Keratinocytes had large nonlobulated nuclei and abundant tonofilaments and desmosomcs. Keratinocytes did not have melanin granules in their cytoplasm. Melanocytes were identified along the basal cell layer (Fig. 2), and presented as clear cells without tonofilaments or desmosomes, but with melanosomes in different stages of maturation.
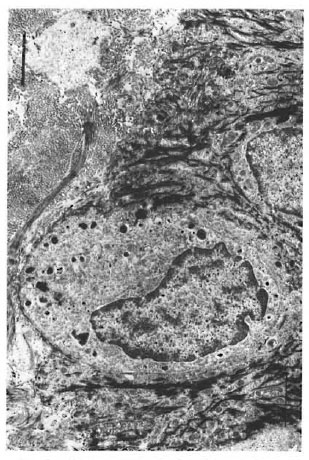
Fig. 2. Melanocyte in the basal layer of the epidermis. Note melanosomes (bar = 2 μm).
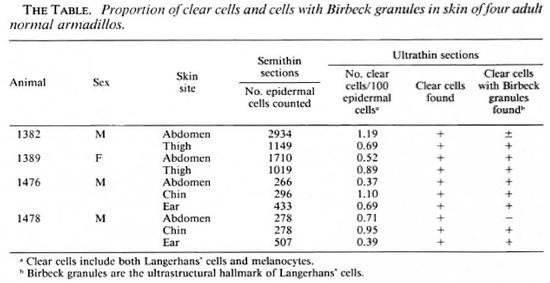
Clear cells with lobulated or indented nuclei and devoid of melanin granules, and the darker staining keratinocytes, were counted in the epidermis by light microscopy in semithin sections (Fig. 1). Relative proportions of these two cell types in the armadillo epidermis are given in The Table. Clear cells in the epidermis were distributed unevenly from the basal cell layer to the upper prickle cell layers. Although clear cells with visible melanin granules were excluded from the count, enumeration of clear cells by light-microscopic evaluation would include both Langerhans' cells and melanocytes with fine melanin not readily visible by light microscopy.
An electron microscopic study showed that there were clear cells without tonolibrils, melanosomes, or desmosomes in their cytoplasm in all ten specimens. The clear cells were round or stellate with long and short cytoplasmic processes. Cytoplasmic organelles of the clear cells, including mitochondria, endoplasmic reticulum and lysosomes, were well formed (Fig. 3). Some cytoplasmic processes extended for short distances between the adjacent keratinocytcs (Fig. 4). There were Birbeck granules with characteristic limiting membranes and periodic stippling in 9 out of 10 clear cells examined (Figs. 5 and 6). In the remaining specimen, there were similar clear cells, but no Birbeck granules were identified in the cytoplasm.
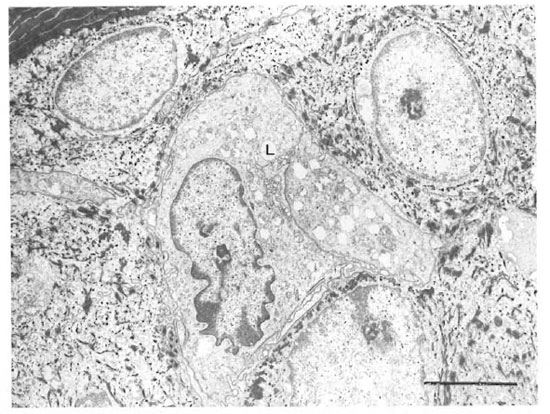
Fig. 3. A Langerhans' cell (L) in the stratum spinosum. Note Birbeck granules in the cytoplasm, the indentednucleus and abundant organelles. The stratum corneum is in the upper left corner (bar = 2 μm).
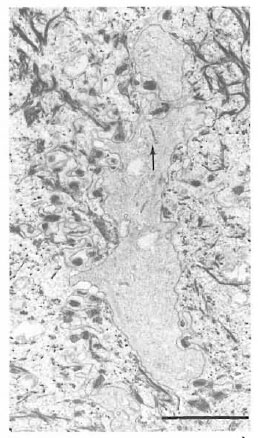
Fig. 4. Birbeck granules (arrow) in a cytoplasmicprocess of a clear cell extending between keratinocytescontaining many tonofilaments (bar = 2 μm).
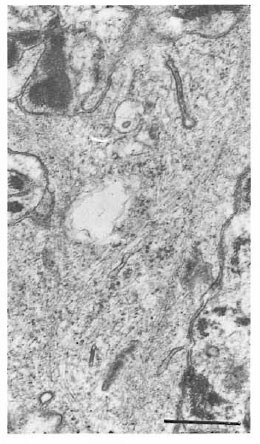
Fig. 5. Birbeck granules in Figure 4 at higher magnification showing granules cut in dilfercnt planes (bar = 0.5 μm).
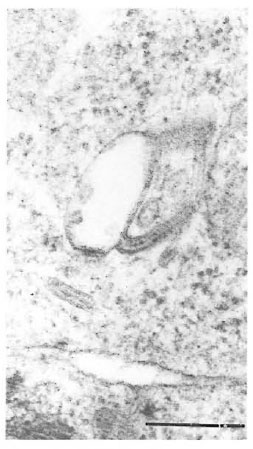
Fig. 6. High magnification of Birbeck granules froman epidermal Langerhans' cell (bar = 0.25 μm).
In one specimen, there were three cytoplasmic fragments of clear cells lying between the basal keratinocytes (Fig. 7). These fragments of clear cytoplasm contained Birbeck granules (Fig. 8). Immediately below the basal lamina in this area, there was a large clear cell with Birbeck granules in the dermal collagen (Fig. 9). No cytoplasmic continuity was seen between the fragments and the dermal cells.
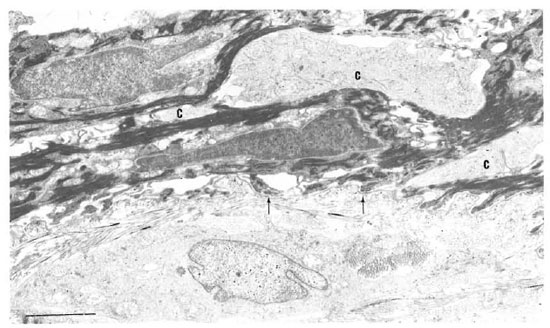
Fig. 7. Birbeck granules in three cytoplasmic fragments (C) between keratinocytes near basement membrane (arrow) of the epidermis. Note Langcrhans' cell surrounded by dermal collagen just beneath basement membrane (bar = 2 μm).
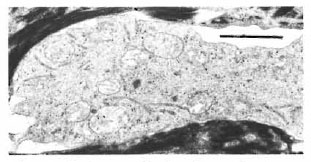
Fig. 8. Higher magnification of a cytoplasmic massin the epidermis of Figure 7 showing many long I3ir-beck granules in the cytoplasm (bar = I μm).
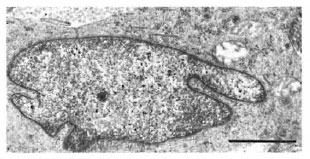
Fig. 9. Portions of Langerhans' cell in the dermisof Figure 7. Note distinctive I3irbeck granules (bar 1 μm).
In a different specimen, there was a crosssection of a cilium in a vesicle-like space in a keratinocyte (Fig. 10). The 9 + 0 pattern of the double tubules of the oligocilium was distinctive. The keratinocyte bearing the cilium was one cell layer above the epidermal basement membrane.
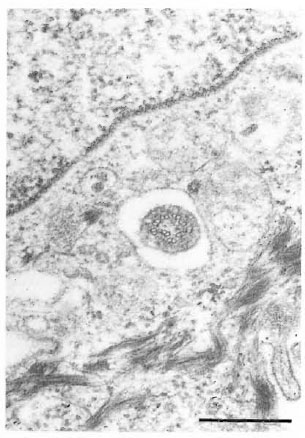
Fig. 10. Cross-section of an oligocilium with char-acteristic 9 + 0 pattern within a vacuole-like space ina keratinocyte (bar = 0.5 μm).
DISCUSSION
The integument of the nine-banded armadillo is composed of two types: a soft, hairy skin over the ventral surface of the head, neck, thorax, abdomen, and proximal by an intermediate form of integument of portions of the extremities, and a scaly, hard thicker skin. carapace over the dorsum of body and head .
The present study shows that the struc and encircling the tail. The ears are covered ture of the epidermis of the armadillo is similar to human epidermis in that it contains keratinocytes, melanocytes and Langcrhans' cells. Keratinocytes of the ninebanded armadillo are arranged in layers similar to that of human skin and, as previously reported, of the seven-banded armadillo (4). The mean relative proportion of clear cells (Langcrhans' cells) and melanocytes is less than 1%. The percentage of Langerhans' cells in the armadillo epidermis, thus, is much lower than in human skin; however, their numbers in different anatomical areas (abdomen, chin, thigh, and ear) are similar. Langerhans' cells have Birbeck granules that are morphologically identical to those in epidermal Langerhans' cells in humans. The evidence suggests that, as in human skin, Langerhans' cells in armadillo skin may migrate across the epidermal basement membrane. The lower proportion of Langerhans' cells in armadillo skin as compared to normal skin is of interest in view of the high susceptibility of nine-banded armadillos to disseminated leprosy when inoculated with Mycobacterium leprae. Studies of Langerhans' cells in human leprosy patients have suggested a decrease in the number and degeneration of Langerhans' cells in lepromatous leprosy patients (8,10). Because the role of Langerhans' cells in the afferent limb of the CMI system is now well understood, the specific immunological defect in both human lepromatous patients and armadillos may be related to the lower numbers of Langerhans' cells. Further studies on the changes in armadillos with experimentally induced or naturally acquired leprosy are needed for a better understanding of this relationship. The baseline data reported here in normal armadillos will be useful in such studies.
Although cilia in basal cells of normal human skin have been reported (l9), the microtubular structure of the cilia in human epidermal cells have not been determined. The cilium of a keratinocyte seen incidentally in normal armadillo skin clearly shows the 9 + 0 pattern of an oligocilium.
Acknowledgment. The authors are grateful to Cynthia Wilson for her generous assistance in processing the manuscript.
REFERENCES
1. Binford, C. H., Meyers, W. M., Walsh, G. P., Storrs, E. E. and Brown, H. L. Naturally acquired leprosy-like disease in the nine-banded armadillo (Dasypus novemcinctus): histopathologic and microbiologic studies of tissues. J. Reticuloendothel. Soc. 22(1977)377-388.
2. Brandt, F., Zhou, H. M. and Shi, Z. R., Kazda, J., Dhople, A. M., Kolk, A. and Schmidt, D. S. The pathology of the eye in armadillos experimentally infected with Mycobacterium leprae Lepr. Rev. 61 (1990)112-131.
3. Breathnach, A. S., Birbeck, M. S. and Everall, J. D. Observations on Langerhans' cells in leprosy. Br. J. Dermatol. 74(1962)243-253.
4. Caparo, A. C. Alias de Histologia del Armadillo de 7-Bandas (Dasypus hybridus). Centro Panamericano de Zoonosis, 1979, pp. 22-29.
5. Friedman, P. S. The immunobiology of Langerhans cells. Immunol. Today 2(1981)124-128.
6. Job, C. K., Harris, E. B., Allen, J. L. and Hastings, R. C. A random survey of leprosy in wild nine-banded armadillos in Louisiana. Int. J. Lepr. 54(1986)453-457.
7. Kirchheimer, W. F. and Storrs, E. E. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. 1. Report of lepromatoid leprosy in an experimentally infected armadillo. Int. J. Lepr. 39(1971)693-702.
8. Liu, J. H., Shi, Y. F., Kong, O. Y., Yang, L. H., Li, W. Z. and Ye, G. Y. Observations on Langerhans' cells in leprosy using monoclonal antibody OK.T6. Int. J. Lepr. 52(1984)524-526.
9. Liu, J. H., Shi, Y. F., Kong, O. Y. and Ye, G. Y. Preliminary observation on Langerhans' cells in leprosy. Int. J. Lepr. 50(1982)316-318.
10. Mathur, N. K., Mangal, H. N., Mathar, D. C. and Agrawal, U. S. Epidermal Langerhans' cells in subtypes of leprosy (delta) Int. J. Lepr. 52(1984)92-93.
11. Narayanan, R. B., Girdhar, B. K. and Desikan, K. V. OKT6+ epidermal Langerhans' cell numbers in DNCB reactions among leprosy patients. Int. J. Lepr. 54(1986)423-426.
12. Epidermis Ultrastructure Purtilo, D. T., Walsh, G. P., Storrs, E. E. and Banks, I. S. The impact of cool temperature on transformation of lymphocytes from humans and armadillos (Dasypus novemcinctus) as related to leprosy. Nature 248(1974)450-452.
13. Purtilo, D. T., Walsh, G. P., Storrs, E. E. and Gannon, C. The immune system of the ninebanded armadillo (Dasypus novemcinctus Linn.). Anat. Rec. 181(1975)725-734.
14. Steinman, R., Hoffman, L. and Pope, M. Control of lymphocyte activation in the skin: maturation and migration of cutaneous dendritic cells. J. Invest. Dermatol. 105(1995)2S-7S.
15. Stingl, G., Tamaki, K. and Katz, S. I. Origin and function of epidermal Langerhans' cells. Immunol. Rev. 53(1980)149-174.
16. Storrs, E. E. The nine-banded armadillo: a model for leprosy and other biomedical research. Int. J. Lepr. 39(1971)703-714.
17. van Voorhis, W. C, Kaplan, G., Sarno, E. N., Horwitz, M. A., Steinman, R. M., Levis, W. R., Nogueira, N., Hair, L. S., Gattas, C. R., Arrick, B. A. and Corn, Z. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N. Engl. J. Med. 307(1982)1593-1597.
18. Walsh, G. P., Meyers, W. M. and Binford, C. H. Naturally-acquired leprosy in the nine-banded armadillo: a decade of experience 1975-1985. J. Leukoc. Biol. 40(1986)645-656.
19. Wilson, R. B. and McWhorter, C. A. Isolated flagella in human skin, electron microscopic observation. Lab. Invest. Dermatol. 80(1983)17-21.
1. H. Zhou, M.D.; Department of Pathology, Qingdao Medical College, Qingdao, China.
2. Z. Shi, M.D., Department of Pathology, Qingdao Medical College, Qingdao, China.
3. A. Mukherjee, M.D., Deputy Director, Institute of Pathology, ICMR, P.O. Box 4909, New Delhi 110029, India.
4. G. P. Walsh, Ph.D., Leonard Wood Memorial, 11600 Nebel Street, Rockville, MD 20852, U.S.A.
5. S. S. Frankel, M.D.; Department of Infectious and Parasitic Disease Pathology, Armed Forces Institute of Pathology, Washington, DC 20306-6000, U.S.A.
6. W. M. Meyers, M.D., Ph.D., Department of Infectious and Parasitic Disease Pathology, Armed Forces Institute of Pathology, Washington, DC 20306-6000, U.S.A.
Reprint requests to Dr. Meyers.
Received for publication on 15 September 1995.
Accepted for publication on 30 November 1995.
1. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Department of the Army or the U.S. Department of Defense.