- Volume 64 , Number 3
- Page: 268–73
Effect of rhulFN-γ treatment inmultibacillary leprosy patients
ABSTRACT
Previous studies have shown that when multibacillary leprosy patients were treated with recombinant human interferon gamma (rhuIFN-γ) for 6-10 months there was an accelerated reduction in the number of acid-fast bacilli in the skin at the site of injection as well as an accelerated bacillary reduction at distal sites. However, this favorable outcome of IFN-γ treatment was associated with the development of erythema nodosum leprosum (ENL). The present study was undertaken to investigate whether rhuIFN-γ-induced bacillary clearance could be disassociated f rom the induction of ENL.rhuIFN-γ was administered together with thalidomide and conventional multidrug chemotherapy to newly diagnosed leprosy patients. During treatment with this combination of drugs, the mean reduction in bacterial load was the same as the reduction observed with chemotherapy alone. Moreover, the inclusion of thalidomide in the treatment regimen was associated with a low frequency of ENL episodes. A second group of leprosy patients, who had already completed 2 years of chemotherapy, were treated with rhulFN-γ only. In those patients who were skin bacilli negative, ENL did not occur during rhuIFN-γ treatment. In contrast, in bacilli-positive patients the frequency of ENL during rhuIFN-γ treatment was higher, as was the occurrence of local erythema and induration. However, rhuIFN-γ treatment without concomitant chemotherapy did not result in a reduction in the bacterial load in the skin of bacilli-positive patients. These findings, taken together, indicate that rhuIFN-γ does not, by itself, accelerate bacterial clearance, but requires concomitant chemotherapy to achieve the accelerated reduction in bacillary load. Thalidomide reduces the frequency of IFN-γ-induced ENL, but also eliminates the IFN-γ-induced bacillary clearance.
RÉSUMÉ
Des études antérieures ont montré que quand des malades lépreux multibacillaires étaient traités avec de 1'interferon-/humain recombinant (IFNγ hur) pendant 6-10 mois, il y avait une réduction accélérée du nombre de bacilles acidos-résistants dans la peau au niveau du site d'injection, ainsi qu'une diminution accélérée de la charge bacillaire dans des sites plus distants. Cependant, cet effet favorable du traitement à l'IFN-γ était associé au développement d'érythème noueux lépreux (ENL). L'étude présente a été entreprise pour rechercher si la diminution de la charge bacillaire induite par l'IFN-γ hur pouvait être dissociée de l'induction de l'ENL.L'IFN-γ hur a été administré en même temps que la thalidomide et une polychimiothérapie conventionnelle à des malades de la lèpre nouvellement diagnostiqués. Au cours du traitement avec cette combinaison de médicaments, la réduction moyenne de la charge bactérienne était la même que celle observée avec la chimiothérapie seule. De plus, l'inclusion de la thalidomide dans le régime thérapeutique était associée à une faible fréquence d'épisodes ENL. Un second groupe de malades de la lèpre, qui avait déjà terminé deux ans de chimiothérapie, a été traité avec de l'IFN-γ hur seul. Chez les patients qui avaient des frottis cutanés négatifs, l'ENL n'est pas apparu au cours du traitement à l'IFN-γ hur. Par contre, chez, les patients avec des frottis cutanés positifs, la fréquence de l'ENL au cours du traitement était plus élevée, ainsi que la survenue d'érythème local et d'induration. Cependant, le traitement à l'IFN-γ hur sans chimiothérapie concommitante n'a pas résulté dans une réduction de la charge bactérienne dans la peau des patients à frottis positifs. Ces observations indiquent que l'IFN-γ hur n'accélère pas par liu-même la disparition des bactéries, mais requiert une chimiothérapie concommitante pour obtenir une réduction accélérée de la charge bactérienne. La thalidomide réduit la fréquence de l'ENL provoqué par l'IFN-γ, mais supprime également l'élimination des bacilles provoquée par l'IFN-γ.
RESUMEN
Previamente se ha demostrado que los pacientes con lepra multibacilar tratados durante 6 a 10 meses con interferon gamma humano recombinants (rhuIFN-γ), muestran una reducción acelerada en el número de bacilos ícido-resistentes en la piel des sitio de inyección así como una reducción bacilar acelerada en sitios distantes. Sin embargo, ests resultado favorable del tratamiento con IFNγ estuvo asociado con el desarrollo de eritema nodoso leproso (ENL). Este estudio se hizo con el fin de investigar si la eliminación de bacilos inducida por el rhuIFN-γ pudiera desasociarse de la inducción de ENL. Para esto, el rhuIFNγ se administró junto con talidomida y con la poliquimioterapia convencional a pacientes con lepra recién diagnosticada. Durante el tratamiento con esta combinación de drogas, le reducción promedio en la carga bacteriana fue la misma que la reducción observada con la quimioterapia sola. Ademís, la inclusión de talidomida en el régimen de tratamiento estuvo asociada con una baja frecuencia de episodios ENL. Un segundo grupo de pacientes que habían completado 2 años de quimioterapia fueron tratados sólo con rhuIFNγ. En aquellos pacientes que no tuvieron bacilos en la piel no hubieron episodios de ENL durante el tratamiento con rhuIFNγ. En contraste, en los pacientes con bacilos la frecuencia de ENL durante el tratamiento con rhuIFNγ fue alta, como también fue alta la frecuencia de eritema e induración locales. Sin embargo, el tratamiento con rhuIFNγ sin quimioterapia no causó ninguna reducción de la carga bacteriana en la piel de Iso pacientes con bacilos. En conjunto, los resultados indican que el rhuIFNγ por si solo no acelera la eliminación de bacilos sino que requiere para ésto del efecto de la quimioterapia concomitante. La talidomida reduce la frecuencia de ENL inducido por el IFNγ pero también bloquea le eliminación de bacilos inducida por esta citocina.It has long been known that interferon gamma (IFN-γ) can activate human monocytes in vitro and thereby stimulate killing of intracellular organisms (7). IFN-γ has, therefore, been used in studies of patients with lepromatous leprosy in whom there is a high bacillary load despite multidrug therapy (MDT). Our previous studies have described the responses of patients with lepromatous leprosy to the intradermal administration of recombinant human IFN-γ (rhuIFN-γ) (2,3,5,6,10,12). Intradermal injection of rhuIFN-γ for 6 days, as an adjunct to MDT, resulted in both a local reduction in the number of acid-fast bacilli (AFB) in the skin at the site of injection and a reduction at distal sites. However, when rhuIFN-γ was administered for the 6-day treatment period and then monthly for 6-10 months, the cytokine was associated with the development of erythema nodosum leprosum (ENL) in 6 of 10 patients (10). Monocytes isolated from the blood of these patients secreted elevated levels of tumor necrosis factor alpha (TNF-α) in response to stimulation with mycobacterial components vitro. Thalidomide treatment of the patients during ENL resulted in a reduction in toxic symptoms and elimination of the lesions of the reactional state. The in vitro addition of thalidomide to cultured monocytes from these patients caused a suppression of TNF-α secretion. These findings suggested that ENL might be associated with enhanced TNF-α production by monocytes and/or accelerated bacterial clearance.
To understand the relationship between rhuIFN-γ-induced bacillary clearance and rhuIFN-γ-induced ENL, multibacillary (MB) leprosy patients were treated with rhuIFN-γ for 6 consecutive days and then monthly for 10 months. Two groups of patients were studied. In one group, the patients were newly diagnosed and on MDT. These patients received rhuIFN-γ and a single 100mg dose of oral thalidomide at the same time as each rhuIFN-γ injection. In the second group, skin bacterial index (BI)-negative and BIl-positive patients who had completed 2 years of MDT received only rhuIFN-γ. Local cellular and systemic responses, the frequency of ENL episodes and the rate of bacillary clearance were evaluated in each group.
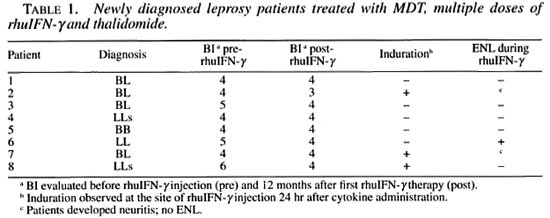
MATERIALS AND METHODS
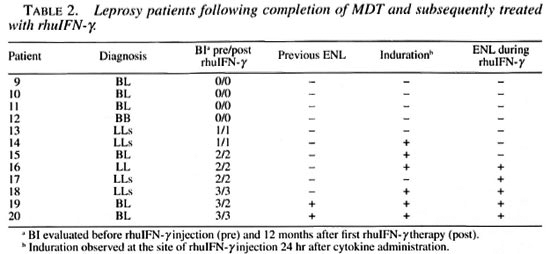
Patient population. Two groups of patients were evaluated at the leprosy clinic, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro, Brazil. In the first group, eight newly diagnosed patients classified, according to the Ridley Jopling classification (9), multibacillary patients on MDT (600 mg rifampin and 300 mg clofazimine monthly, supervised and 100 mg dapsone and 50 mg clofazimine daily, unsupervised) were included in this study. Of these, 4 were classified as borderline lepromatous (BL), 2 subpolar lepromatous (LLs), 1 polar lepromatous (LL), and 2 midborderline (BB) (Table 1). The second group consisted of 12 patients who had completed 24 months of MDT and were under clinical surveillance in the absence of MDT. In this group, 6 were classified as BL, 4 were LLs, 1 was LL and 1 was BB. Four of these patients (patients 9-12) had a negative skin BI (BI = 0), and in 8 patients (patients 13-20) the BI was still positive (mean ± S.D. = 2.1 ± 0.8) (Table 2). Two patients in the treated group (patients 19 and 20) had a previous history of reactional episodes (ENL). None of the other patients had any reactional manifestation before the beginning of the study.
huIFN-γ injections
rhuIFN-γ(Boehringer-Ingelheim, Ingeheim am Rhein, Germany) was used for these studies. The lyophilized cytokine (specific activity 2 x 107 U/mg of protein) was reconstituted in pyrogen-free sterile water. After written informed consent, patients were injected intradermally on the back with a single dose of 30 µ g of rhuIFN-γ daily for 3 days followed by 3 days of respite and then three additional daily injections of 30 µ g rhuIFN-γ, for a total of six injections. One month after the last dose of 30 µ g, patients received a single dose of 100 µ g injected intradermally in the back; this continued monthly for a total of 10 monthly injections of 100 µ g of rhuIFN-γ.
Patient evaluation
Patients were evaluated clinically and bacteriologically before the injections, after the 1st, 6th, and 10th months of treatment, and then at 12 months after the initiation of the study. Induration at the site of injection was recorded 24 hr after each 30 µg injection, and the patients were questioned for the presence of any symptoms and/or induration at the injection site when they came to receive the next monthly dose of the cytokine.
Thalidomide treatment
The eight newly diagnosed patients (patients 1-8; Table 1) who did not have ENL received a single 100-mg tablet of thalidomide orally at the same time as each of the injections of rhulFN-γ.
Quantitation of bacterial load
The BI is a numerical microscopic estimate of the bacterial burden in patients diagnosed with leprosy (the number of AFB per 100x field oil immersion) (8). Slit-skin smears for the Bl determination were taken from six anatomic sites (usually earlobes, elbows, a knee, and a lesion).
RESULTS
Newly diagnosed MB leprosy patients treated with adjunctive therapy with rhuIFN-γ and thalidomide
Clinical response. When patients with newly diagnosed MB leprosy were treated with rhuIFN-γ and thalidomide as an adjunct to MDT, there was a local erythematous and indurative response in only 3 of the 8 patients treated with the cytokine. None of the patients treated with rhuIFN-γ and thalidomide exhibited the mild systemic symptoms associated with rhuIFN-γ treatment reported previously (10). Malaise, fatigue, low-grade fever, nausea or weight loss were not observed.
Changes in BI. There was at least a 1 -log reduction in the BI in 4 of 8 newly diagnosed patients who received adjunctive treatment with rhuIFN-γand thalidomide (Table 1). For the group as a whole, a mean 0.6-log reduction in the BI was observed over the 12-month period of treatment and evaluation. This is analogous to the reduction in the BI usually observed in response to MDT alone during 1 year of treatment.
Development of ENL. The systemic inflammatory reaction to rhuIFN-γ injection observed in the majority of those patients treated with rhuIFN-γ in previous studies (10) was not observed when the cytokine was administered together with 100 mg of thalidomide. Only 1 patient of the 8 in this group of newly diagnosed patients developed ENL during the 12 months of this study. Two patients developed neuritis in the absence of any ENL manifestations. The neuritis was observed in 2 of the 3 patients who had reported local induration at the site of rhuIFN-γ injection.
Leprosy patients after MDT treated with rhuIFN-γ therapy alone
Clinical response. In this group, patients had completed 2 years of MDT before entering the study. Following rhuIFN-γ treatment according to the same protocol described above, without concomitant MDT, two different types of clinical responses were observed. Of the BI-negative patients (patients 9-12; Table 2), none reported local erythema or induration at the site of the rhuIFN-γ injection. However, all of the BI-negative patients experienced systemic symptoms including fever, headache, and nausea, 0 to 4 hr after the rhuIFN-γ injections. One patient reported a transient orchitis (which could be an indication of mild ENL) at the end of the study. In contrast, 6 of the 8 Bl-positive patients in this group developed erythema and induration within 24 hr at the site of the rhuIFN-γ injection. During the study, all of the Bl-positive patients reported some mild clinical symptoms including nausea, headache, low-grade fever, and fatigue following the rhuIFN-γ injections.
Changes in BI. In contrast to the results observed with the group of newly diagnosed patients, 7 of 8 patients in group II who were BI positive showed no changes in the BI during 12 months of treatment with rhuIFN-γ in the absence of MDT. Patients in group II who were BI negative at the inception of the study remained BI negative.
Development of ENL. BI-negative patients who received rhuIFN-γ without MDT for 12 months did not develop ENL during the course of the study. However, 5 of 8 of the Bl-positive patients in this group did develop ENL following rhuIFN-γ treatment.
DISCUSSION
Two groups of patients with MB leprosy were treated with rhuIFN-γ in this study. Group I consisted of newly diagnosed patients on MDT who received rhuIFN-γ simultaneously with thalidomide in conjunction with continued MDT. Group II consisted of patients who had completed 2 years of MDT and who now received rhuIFN-γ alone without either MDT or thalidomide.
Our studies demonstrate that IFN-γ-induced bacillary clearance and IFN-γ-induced ENL, which is putatively mediated by TNF-α production, cannot be maintained independently of each other. When rhulFN-γ was administered to patients in group I, together with thalidomide which presumably inhibits TNF-α production, the mean reduction in the bacterial load was not different from the reduction usually observed with MDT therapy alone (The Fig. A). IFN-γcombined with MDT has previously been shown to induce an accelerated clearance of bacteria ('"). The present observation suggests that thalidomide prevents the induction of enhanced bacterial clearance by IFN-γ treatment. Thalidomide does not interfere with bacterial clearance which occurs in response to MDT. Thus, the inflammatory component induced by IFN-γtreatment (presumably TNF-α) which is associated with both ENL and the accelerated reduction in bacterial load is inhibited by thalidomide.
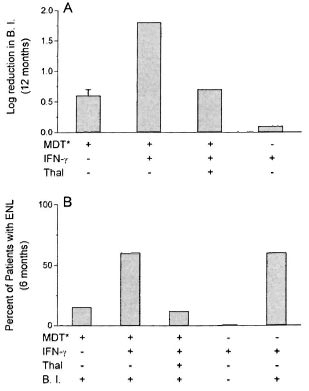
The figure. Effect of treatment on rate of reduction in skin bacillary load (A) and on development of ENL (B) in leprosy patients. Results are expressed asmean log reduction in BI during 12 months (A) andpercent of patients who developed ENL during 6 months of treatment (B) with (+) or without (-) MDT, IFN- y and thalidomide (Thal) in patients with (+) orwithout (-) bacilli (BI) in their skin at the start of the study. * = See Materials and Methods for doses andfrequency of treatment. Number of patients in eachgroup listed in Tables I and 2 and in Reference.
Thalidomide has been shown to inhibit the production of TNF-α (4,10,11). It may be that bacterial clearance depends in part on TNF-α production by monocytes activated by either endogenous or exogenous IFN-γ (1). TNF-α may render infected macrophages in the skin better capable of bacteriocidal activity. It appears that the effect of exogenous IFN-γoccurs only when TNF-α is induced. However, because TNF-α production causes significant toxicities (ENL) in these patients, the therapeutic use of IFN-γ may be limited. ENL may have more deleterious clinical consequences than a slower bacterial clearance obtainable with MDT alone.
In the group of newly diagnosed patients, thalidomide treatment in conjunction with MDT and IFN-γ was associated with low frequency in the occurrence of ENL (The Fig. B). Previous studies have shown that when MB leprosy patients are treated with rhuIFN-γ and MDT, approximately 60% develop ENL during the first 6 months of the treatment period (10). In contrast, the addition of thalidomide to this regimen reduced the frequency of ENL episodes to 12% in the same time period. This lower frequency of ENL is comparable to the frequency observed in patients treated with MDT alone. The present results provide additional evidence for a role for TNF-α in the induction of ENL.
In the second group of patients, who had already completed MDT before the start of this study, the presence or absence of Mycobacterium leprae in the skin (BI) appeared to be associated with the frequency of development of ENL. In BI-negative patients, ENL did not occur during rhuIFN-γ treatment despite the appearance of systemic symptoms following rhuIFN-γ injections. These patients did not develop erythema or induration at the site of IFN-γ injections. In BI-positive patients, the frequency of ENL was much higher as was the frequency of local erythema and induration. The local cutaneous response appeared to precede development of ENL, while the mild systemic symptoms did not necessarily indicate development of ENL. Thus, it appears that the presence of M. leprae in the skin is required for ENL induction.
It is of interest to note that rhuIFN-γ treatment without concomitant MDT did not result in a reduction in the bacterial load in the skin of BI-positive patients. This finding suggests that IFN-γ does not, by itself, accelerate bacterial clearance. rhuIFN-γ treatment together with MDT and thalidomide also does not accelerate bacterial clearance. Since thalidomide inhibits TNF-α production (11) it appears that TNF-α is required for accelerated bacterial clearance. Treatment with rhuIFN-γ, although it can lead to reduction in the bacillary load when administered together with MDT, has a significant risk of causing ENL. Therefore, further large-scale patient studies which would be required to expand and to confirm the role of exogenous IFN-γ in the possible mechanism of ENL induction are not recommended.
Acknowledgment. These studies were supported in part by NIH grant AI-33124 and by the Celgene Corporation. We thank Dr. Victoria Freedman for help in writing the manuscript. Marguerite Nulty for typing the manuscript, and Judy Adams for help with preparation of the figures.
REFERENCES
1. Bermudez, L. E. and Kaplan, G. Recombinant cytokines for controlling mycobacterial infections. Trends Microbiol. 3(1995)22-26.
2. Kaplan, G., Mathur, N. K., Job, C. K., Nath, I. and Cohn, Z. A. The effect of multiple IFN-γ injections on the disposal of Mycobacterium leprae. Pnas 86(1989)8073-8077.
3. Kaplan, G., Nusrat, A., Sarno, E. N., Job, C. K., Mcelrath, J., Porto, J. A., Nathan, C. F. and Cohn, Z. A. Cellular responses to the intradermal injection of recombinant human interferon-γ in lepromatous leprosy patients. Am. J. Pathol. 128(1987)345-353.
4. Makonkawkeyoon, S., Limson-Pobre, R. N. R., Moreira, A. L., Schauf, V. and Kaplan, G. Thalidomide inhibits the replication of human immunodeficiency virus type 1. Pnas 90(1993)5974-5978.
5. Nathan, C. F., Kaplan, G., Levis, W. R., Nusrat, A., Witmer, M. D., Sherwin, S. A., Job, C. K., Horowitz. C. R., Steinman, R. M. and Cohn, Z.A. Local and systemic effects of low doses of recombinant interferon -γ after intradermal injection in patients with lepromatous leprosy. New Engl. J. Med.315(1986)6-15.
6. Nathan, C F., Squires, K., Griffo, W., Levis, W., Varglese, M., Job, C. K., Nusrat, A. R., Sherwin, S., Rappaport, S. Sandes, E., Burk-Hardt, R. A. and Kaplan, G. Widespread intradermal accumulation of mononuclear leukocytes in lepromatous leprosy patients treated systemically with recombinant interferon-γ. J. Exp. Med. 172(1990)1509-1512.
7. Nathan, C. F. and Yoshida, R. Interferon-γ. In: Inflammation: Basic Principles and Clinical Car-relates. Gallin, J., Goldstein, I. and Snyderman, R., eds. New York: Raven Press, Ltd., 1988, pp. 229-251.
8. Ridley, D. S. and Hilson, G. R. F. A logarithmic index of bacilli in biopsies I. Method. Int. J. Lepr. 35(1967)184-186.
9. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
10. Sampaio, E. P., Moreira, A. L., Sarno, E. N., Malta, A. M. and Kaplan, G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J. Exp. Med. 175(1992)1729-1737.
11. Sampaio, E. P., Sarno, E. N., Gallilly, R., Cohn, Z. A. and Kaplan, G. Thalidomide selectively inhibits TNFα production by stimulated human monocytes. J. Exp. Med. 173(1991)699-703.
12. Samuel, N. M., Grange, J. M., Samuel, S., Lucas, S., Owilli. O. M., Adalla, S., Leigh, I. M. and Navarretta, C. A study of the effects of intradermal administration of recombinant gamma interferon in lepromatous leprosy patients. Lepr. Rev. 58(1987)389-400.
1. M.D., Ph.D.
2. M.D.
3. M.D., Leprosy Laboratory, Oswaldo Cruz Institute, Fiocruz, Manguinhos, Rio de Janeiro, Brazil 21045.
4. Ph.D., Laboratory of Cellular Physiology and Immunology, The Rockefeller University, 1230 York Avenue, New York, New York 10021, U.S.A.
Reprint requests to Dr. Kaplan at above address or FAX = 212-327-8875; email = kaplang@rockvax.rockefeller.edu.
Received for publication on 17 November 1995.
Accepted for publication in revised form on 22 February 1996.