- Volume 64 , Number 3
- Page: 274–81
Microbial colonizers in leprosy skin ulcers and intensity of inflammation
ABSTRACT
The microflora of 55 patients with leprosy skin ulcers was studied and related to a weighted inflammatory score (IS). The control group consisted of 18 ulcers with different underlying pathology. Leprosy ulcers were characterized by the exclusive presence of two types of branching gram-positive rods; a particular interesting proposal is that Mycobacterium leprae share common antigens with these unusual "leprosy ulcer associated" organisms and group G betahemolytic streptococci. In the leprosy group, corynebacteria and branching rods accounted for 97% of gram-positive bacilli and Bacillus species constituted only 3%. In the control group, B. species formed 50% of gram-positive rods; the rest were corynebacteria (p = 0.03). In the leprosy group, one third of the gram-positive bacteria were branching rods; none of them was acid fast. Ten of them were identified asAr canobacterium haemolyticum, and the remaining 7 could not be identified. The IS of leprosy patients was lower than in the control group. The presence of more than two species of facultative or aerobic gram-negative rods or single species of pyogenic gram-positive cocci correlated with a high IS. The presence of two or more different pyogenic cocci resulted in a lower IS. Further studies into the nature of leprosy-unique organisms as well as the inflammation inhibition factors in mixed infections are warranted. It is recommended that management of ulcers should consist of the application of local disinfection and early treatment of episodes of inflammation with a combination of fluoroquinolone and penicillin.RÉSUMÉ
On a étudié la microflore de 55 patients avec des ulcères cutanés lépreux, en relation avec un score inflammatoire (SI) pondéré. Le groupe témoin se composait de 18 ulcères avec diverses maladies sous-jacentes. Les ulcères lépreux se caractérisaient par la présence exclusive de deux types de baguettes raméfiées gram-positives; une proposition particulièrement intéressante est que Mycobacterium leprae partage des antigènes communs avec ces organismes inhabituels "associés aux ulcères lépreux" et des streptocoques beta hémolytiques du groupe G. Dans le groupe lèpre, les eorynebactéries et les baguettes raméfiées comptaient pour 97% des bacilles gram positifs et Bacillus species seulement pour 3%. Dans le groupe témoin, B. species formait 50% des baguettes gram positives; le reste était des coryne bactéries (p = 0.03). Dans le groupe lèpre, un-tiers des bactéries gram positives était des gabuettes raméfiées; aucune d'entre elles ne résistait à l'acide. Dix d'entre elles ont été identifiées en tant que Arcanobacterium haemolyticum , et les 7 autres n'ont pu être identifiées. Le SI des patients lépreux était plus faible que celiu du groupe témoin. La présence de plus de deux espèces de baguettes gram négatives facultatives ou aérobiques ou d'une seule espèce de coques gram positifis pyogènes était associé à un SI élevé. La présence de deux coques pyogéniques différents ou plus résultait dans un SI plus faible. Des études ultérieures se rapportant à la nature de ces organismes uniques à la lèpre ainsi que sur les facteurs d'inhibition de l'inflammation dans les infections mixtes vont être féalisées. On recommande que le traitement des ulcères consiste en l'application d'une désinfection locale ainsi que le traitment précoce des épisodes d'inflammation par une combinaison de fluoroquinolone et de pénicilline.RESUMEN
Se estudió la microflora de las úlceras de piel de 55 pacientes con lepra y se relacionó con el registro del peso inflamatorio (l'I). El grupo control estuvo conformado por 18 pacientes con úlceras de patologías diversas no leprosas. Las úlceras de la lepra se caracterizaron por la exclusiva presencia de 2 tipos de bacilos ramificantes Gram-positivos. Se propone que Mycobacterium leprae comparte antígenos comunes con estos organismos asociados con las úlceras de la lepra y con estreptococos beta-hemolíticos del grupo G. En el grupo de lepra las corinebacterias y los bacilos ramificantes constituyeron el 97% de los bacilos Grampositivos y las especies del género Bacillus sólo el 3%. En el grupo control, las especies de Bacillus formaron el 50% de los bacilos Gram-positivos, el resto fueron corinebacterias (p = 0.03). En el grupo de lepra, un tercio de las bacterias Gram-positivas fueron bacterias ramificantes; ninguna de ellas fue ácido-resistente. Diez de ellas fueron identificadas como Arcanobacterium haemolyticum y los 7 restantes no pudieron identificarse. El PI en los pacientes con lepra fue más bajo que el PI en el grupo control. La presencia de más de 2 especies de bacilos facultativios o aeróbicos Gram-negativos o de especies únicas de cocos piógenos Gram-positivos correlacionó con un PI elevado. Se requieren más estudios sobre la naturaleza de los organismos asociados a la lepra y sobre los factores inhibitorios de inflamación en las infecciones mixtas. Se recomienda que el manejo de las úlceras incluya la desinfección local y el tratamiento temprano de los episodios de inflamación con una combinación de lluoroquinolona y penicilina.Mycobacterium leprae is the causative agent of leprosy, a disease in which the clinical presentation varies with the patient's immune response (15). Skin lesions are seen at the lepromatous as well as the tuberculoid polesof the disease spectrum. Although the underlying immune pathology of the lesions is different across the spectrum, chronic ulceration can develop at both polesof leprosy (3). These ulcers become infected with bacteria other than M. leprae and show recurrent episodesof cellulitis of the surrounding tissues as well as osteitis of underlying bone. This, in turn, results in deformities which disable patients and contribute to the stigma associated with the disease (1). Early and adequate treatmentof these episodes of inflammation could interrupt this sequenceof events.
There are several reports (4-6) which show that chronic skin ulcers in leprosy patients are colonized with gram-positive rods which have not been observed in chronic-skin ulcers of different etiology. However, there are no studies which compare culture resultsof leprosy-related and other skin ulcers in the same population. Also, no attempt has been made to relate colonizing species with inflammation.
This study compares organisms present in leprosy skin ulcers with those present in skin ulcers of different etiologies, and correlates this with the severity of inflammation. Susceptibility patterns of these organisms are reported and the possible contribution of antimicrobial treatment toward prevention of deformity is discussed.
PATIENTS AND METHODS
Patients were recruited from the population attending Marie Adelaide Leprosy Centre (MALC) in Karachi, Pakistan. All patients with ulcers, from whom informed consent was obtained in the period between October 1991 and October 1992, were enrolled. Patients with nonleprosy skin ulcers of varying durations and not on antimicrobial treatment for at least 2 weeks were recruited as a control group at the surgery clinic of Civil Hospital, Karachi.
Inflammatory scores (IS) were measured based on the method of Ehrenkranz, et al. (7). This included amount and character of drainage, extent of cellulitis, presence of foul odor and presence of necrosis. Observations were scored as follows: drainage on dressing = 0; minimal, moderate and copious drainage in ulcer bed =1, 2 and 4, respectively; serous, sanguinous, sanguinopurulent and purulent drainage = 0, 1, 3 and 4, respectively; widest cellulitis width of < 1 cm, > 1 to 2 cm, > 2 to < 3 cm, > 3 to < 4 cm and > 4 cm = 0, 1,2,3 and 4, respectively; no foul odor = 0; foul odor = 1; no necrosis = 0; necrosis in ulcer margin - I; necrosis in ulcer margin and base = 2. The IS value is the sum of these scores and ranges from 0 to 15.
Ulcers were sampled by slightly modifying the irrigation-aspiration method (7). In brief, at least 1 ml of sterile 0.85% sodium chloride without preservatives is irrigated under the margin of the ulcer with a needleless 5-ml syringe. Excess fluid is removed with a sterile gauze pad. The ulcer margins are massaged circumf'erentially with a sterile cotton swab. Alter this, a second irrigation is performed using 1 ml of saline and a new syringe. Without removing the excess fluid, the ulcer margin is massaged again after which the ulcer base and margins are swabbed with a sterile cotton-tipped swab which is immediately wrung into 1 ml of brain-heart infusion (BHI) broth for staining and culture.
Smears of broth aspirate suspensions were stained by means of Gram as well as Kinyoun stain. Cultures were performed on a 7% sheep blood agar, cysteine lactose electrolyte-deficient medium (CLED), an isovitalex enriched chocolate blood agar and a nalidixic acid (10 mg/l) mecillinam (1 mg/1) bilayered 7% sheep blood agar. All plates were incubated at 37ºC; the latter two in a CO2 incubator. In addition, a Sabouraud agar with 10 mg/1 gentamicin and a Columbia agar with 2% Tween 80 and nalidixic acid 10 mg/1 were inoculated and incubated at 30ºC. Two sets of Lowenstein-Jensen slants incubated for 12 weeks at 30ºC and 37ºC, respectively, were used for the culture of mycobacteria. The presence of anaerobes was evaluated on microscopy of Gram-stained smears, based on multiplicity of morphological forms, intracellular location, pale staining of gram negatives and size as well as characteristic appearance.
Identification of organisms was done by means of established methods (10) and based on the latest edition of Bergey's Manual of Determinative Bacteriology (12,16).
Minimal inhibitory concentrations (MIC) for antimicrobial agents were measured by means of the agar dilution method on isosensitest agar with an inoculum of 104 cfu/spot (14).
Mycobacterial culture media were obtained from DIFCO Laboratories, Detroit, Michigan, U.S.A.; all others were obtained from Oxoid, Basingstoke, Hampshire, England.
Fisher's exact test was used to determine the significance of differences between leprosy and control ulcers. Mann-Whitney and Kendall rank order correlation coefficient tests were used for comparison/correlation between groups.
RESULTS
Fifty-five patients with leprosy skin ulcers and 18 control patients were enrolled in the study. Five of the study patients had the tuberculoid form of leprosy, 13 the lepromatous and 37 had borderline leprosy. Thirty-nine patients, from whom a reliable history could be taken, had suffered from the disease for many years (mean duration 14.2 years). At the time of enrollment, 30 patients were not on any treatment, 10 were on dapsone, 1 was on clofazimine, and 14 were on the multidrug therapy (MDT) regimen of clofazimine, dapsone and rifampin. Only three patients had never been on antileprosy medications; all the others had been treated previously with different regimens.
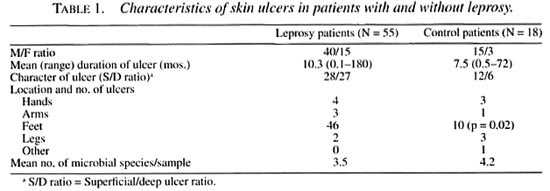
Ulcer characteristics. Table 1 compares the characteristics of the skin ulcers of leprosy patients with those of the control group. In leprosy patients, ulcers were present predominantly on the feet compared with the nonleprosy patients. A higher percentage of leprosy ulcers were deep and of longer duration than the control ulcers. Seven of the 18 nonleprosy patients suffered from post-traumatic ulcers, 6 had underlying diabetes mellitus, and 1 ulcer followed an insect bite; definite etiology could not be confirmed in 4 cases.
Ulcer microflora. When the microbial flora was compared in the study and control groups, no difference was found in the mean number of microbial species per sample between the two groups (Table 1). Table 2 shows that leprosy patients had significantly more beta-hemolytic streptococci. Group G streptococcus was exclusively present in ulcers of leprosy patients (9.7% of isolates). Gram-positive rods accounted for 29.3% of all isolates from the ulcers of leprosy patients and 25% in the control patients. The distribution of species from the genera Corynebacterium and Bacillus was different in the two patient groups. In the leprosy group, corynebacteria and branching rods accounted for 97% of gram-positive bacilli and Bacillus species constituted only 3% of the group. In the control patient group, Bacillus species formed 50% of gram-positive rods; the rest were corynebacteria (p = 0.03). In the leprosy group, one third of the gram-positive bacilli were branching rods. None of these were acid fast. Ten strains were identified as Arcanobacterium haemolyticum; the remaining 7 could not be identified with the methods used.
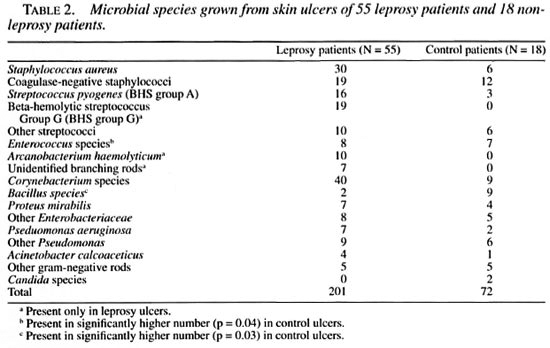
Skin ulcers of patients in the control group grew yeast (two samples) and higher numbers of species of enterococci (p = 0.04) and Enterohacteriaceae (p = 0.08) compared to the leprosy group. Moreover, no branching rods were isolated from the control ulcers. Anaerobes (on microscopy) were found with the same frequency in the study (18%) and in the control (17%) groups. No mycobacteria could be cultured in either group.
Patients with leprosy had an inflammatory score (IS) which varied between 0 to 13 (median of 2; mean score of 3). This was significantly (p = < 0.001) lower than in the control group which showed a variation in IS between 1 and 10 (mean 5.5; median 6). No correlations were found between the IS and age of the patients, durationof ulcers, or type and durationof leprosy. The four control group ulcers, which etiology could not be ascertained, had a median IS of 1.5 with a meanof 3.25 (range 1-9); this value is similar to that in the leprosy group.
In the leprosy group, the numberof species per sample appeared to be directly related to the IS (Fig. 1); no such correlation was found for the nonleprosy control patients. The IS was higher in leprosy ulcers from which gram-negative rods were isolated (p = 0.007; Fig. 2). Also, the presence of either Staphylococcus aureus or one of the hemolytic streptococci resulted in a higher IS. However, if a combination of two or more of these species of pyogenic cocci was present in the ulcer, the IS significantly dropped (p = 0.017; Fig. 3).
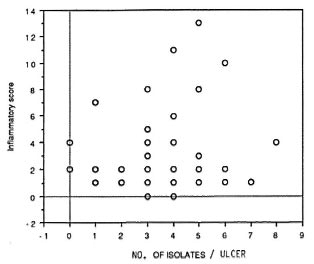
Fig. 1. Higher inflammatory scores appear to beassociated with higher numbers of isolates from the sample.
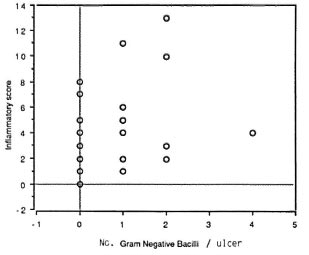
Fig . 2. There is a positive correlation (Tau =0.228; p = 0.014) between gram-negative bacilli andinflammatory score.
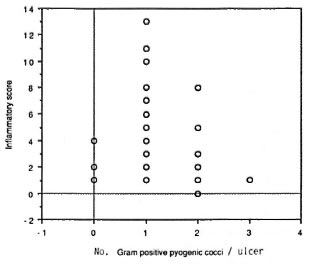
Fig . 3. There is a negative correlation (Tau = 0.22; p = 0.018) between the presence of two or more pyo-genic cocci and inflammatory score.
The presence in the leprosy group exclusively of the branching gram-positive rods ( A. haemolyticum and an unidentified species) was not related to the IS. However, the presence of these organisms in combination with gram-negative rods was associated with a higher IS as compared to gram-negative rods only (p = 0.005). Such an effect was not seen with other bacteria. No difference was found between the IS and the presence of A. haemolyticum or the IS and the unidentified branching rods.
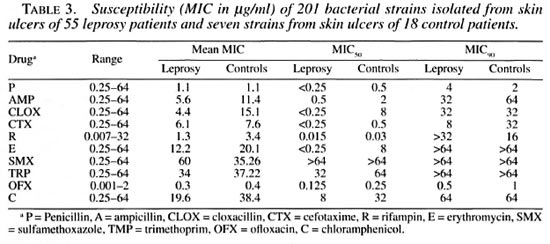
Table 3 shows the MICs of the isolates from both groups of ulcers. Resistance to the sulfonamides was seen in almost all isolates. Both S. aureus and the nonaureus staphylococci (CNS) had a significantly higher mean MIC for rifampin. This difference was not observed with group A streptococci. However, streptococci other than groups A and G were also more resistant to rifampin. The highest mean MIC for rifampin as compared to all other gram-positive bacteria was seen in group G streptococci. These differences were observed at MIC90 but not at the MIC50 level, indicating that this was caused by a few strains with high levels of resistance rather than resistance in all strains.
DISCUSSION
One of the major aims of this study was to identify a relationship between clinically detectable inflammatory changes and the presence of specific bacterial species in the skin ulcers of leprosy patients. The Sampling technique used in this study has been shown to be in decubitus ulcers as representative for culture as a biopsy (7).
We observed that the microflora of skin ulcers in leprosy patients was different from the flora of nonleprosy skin ulcers. Both leprosy and control patients were from similar socioeconomic backgrounds and environmental differences are, therefore, unlikely to be accountable for those differences. The significant observation was the isolation of gram-positive branching bacilli in leprosy ulcers, more than half of which were identified as A. haemolyticwn. This organism has not been reported previously from leprosy ulcers. It has been described as a case report in relation to a post-traumatic skin ulcer and as a causative agent of outbreaks of tonsillo pharyngitis (9). Group G beta-hemolytic streptococci also were exclusively isolated from leprosy ulcers. Certain species from the genus Corynebacterium have been described as unique for leprosy patients (6). The branching rods from our study patients which remained unidentified could be similar to these leprosy-derived corynebacteria. Cell-wall composition and DNA analysis of these strains may provide the answer (2,11).
It has been postulated that the presence of certain gram-positive rods in leprosy ulcers is explained by their antigenic relatedness to M. leprae (8,13). Tolerance to common antigens perhaps allows these organisms to colonize the leprosy ulcers. This would imply that all of the isolates which are unique to leprosy ulcers may share some antigenic features with M. leprae. At least two different organisms have been reported with this characteristic (8,13) but neither one has been classified officially. From the present study we may postulate that A. haemolyticum, unidentified branching rods, and group G streptococci probably also belong to this group. One would expect differences in colonization with these organisms at the poles of the spectrum of leprosy depending on the patient's immune status. We could not establish such a relationship because of the small numbers of patients with tuberculoid and lepromatous leprosy. To resolve the issue of shared antigens, a comparative study between these organisms and M. leprae will be required.
Isolation of group G streptococci from leprosy patients only could reflect cross-infection in the health care facility from which the patients were recruited. The same may be true for A. haemolyticum and other branching rods. In such a situation, there would be a clustering of cases in time. However, these organisms were isolated throughout the year. Also, if spread from patient to patient or from health care worker to patient was a major factor, one would expect to find the more virulent S. aureus and group A streptococci. Hence, nosocomial outbreaks cannot be held responsible for our observations.
Another possible reason for the differences in colonization of skin ulcers of leprosy and control patients could be the selection of organisms resistant to the drugs used for the treatment of leprosy. Therefore, dapsone (sulfonamide) and rifampin resistance was examined. All branching rods and group G streptococci were resistant to sulfonamides but so were all of the other organisms in the leprosy as well as the control group. Resistance to rifampin showed a more complicated picture. There was a significantly higher level of resistance among different groups of bacterial isolates from leprosy patients, including group G streptococci. This difference is, however, based on a broad range of MIC values and not on higher MICs for all isolates. The branching rods were fully susceptible as were 15 out of the 19 group G streptococci; the other 4 showed high-level resistance. These four were responsible for the high mean MIC and high MIC90 values. Susceptibility tests for clofazimine were not performed. Although resistance to the antileprosy drugs was found with higher frequency in the leprosy group, it does not provide an explanation for the observed difference in colonizing species. The presence of drug-susceptible strains (branching rods) cannot have been due to the selective pressure of the antibiotics used.
Leprosy ulcers showed a low mean inflammatory score (IS) as compared to the control group, the IS of the latter being in accordance with the findings of others (7). The presence of pyogenic streptococci together with S. aureus resulted in a lower IS than either of these separately, while the presence of A. haemolyticum or unidentified branching rods together with facultative gram-negative bacilli enhanced inflammation. The mechanism of these bacterial interactions is unclear and warrants further study. If a high IS is related to the development of cellulitis and osteitis of adjacent tissue, leading to systemic disease and deformity, regular IS evaluation with timely intervention could prevent this. Based on observations of this study, the aim should be continuous ulcer care to reduce the number of colonizing bacteria and antimicrobial treatment when there is a rise in the inflammatory reaction. The number of colonizing organisms also can be kept low by applying disinfecting dressings, while the choice of antimicrobial drugs should focus on enterobacteria as well as pyogenic cocci. A combination of a fluorinated quinolone with penicillin would be adequate. However, the efficacy of such an approach has yet to be established.
Acknowledgment. This study was financially supported by LEPRA, the British Leprosy Relief Association, through a grant to AWS.
REFERENCES
1. Antia, N. H. The people we fail to reach. Lepr. Rev. 48(1977)155-156.
2. Antonie, I., Coene, M. and Cocito, C. Size and homology of the genomes of leprosy-derived corynebacteria, Mycobacterium leprae and other corynebacteria and mycobacteria. J. Med. Microbiol. 27(1988)45-50.
3. Bechelli, L. M. and Martinez Dominquez, V. The leprosy problem in the world. Bull. WHO 34(1966)811-826.
4. Brown, S., Lanelle, M. A., Asselineau, J. and Barksdale, L. Description of Corynebacterium tuberculostearicum sp. nov., a leprosy-derived corynebacterium. Ann. Microbiol (Institut Pasteur) 135B(I984)251-267.
5. Chakrabarty, A. N., Dastidar, S. G., DAS, S. and Chaudhury, S. K. Repeated isolation of Nocardia -like organisms from multibacillary leprosy. Indian J. Lepr. 59(1984)247-262.
6. Cocito, C. and Delville, J. Properties of microorganisms from human leprosy lesions. Rev. Infect. Dis. 5 (1983) 649-657.
7. Ehrenkranz, N. J., Alfonso, B. and Nezenberg, D. Irrigation-aspiration for culturing draining decubitus ulcers: correlation of bacteriological findings with a clinical inflammatory scoring index. J. Clin. Microbiol. 28(1990)2389-2393.
8. Gueur, M. C, Harboe, M., Fontain, F., Delville, J. and Cocito, C. Comparison of the cytoplasmic antigens of leprosy-derived corynebacteria and some mycobacteria. Scand. J. Immunol. 17(1983)497-506.
9. Hoosen, A. A., Rasool, M. N. and Roux, L. Post traumatic ankle joint infection with Arcanobacterium haemolyticum: a case report. J. Infect. Dis. 162(1990)780-781.
10. Isenberg, H. D. Clinical Microbiology Procedures Handbook. Washington, D.C.: American Society for Microbiology, 1992.
11. Janczura, E., Abou-Zeid, C, Gailly, C. and Cocito, C. Chemical identification of some cell wall components of microorganisms isolated from human leprosy lesions. Zentralbl. Bakteriol. 251(1981)114-125.
12. Krieg, N. R. and Holt, J. G. Bergey's Manual of Systemic Bacteriology Vol. I. Baltimore: Williams and Wilkins, 1984.
13. Laub, R., Delville, J. and Cocito, C. Immunological rclatcdness of ribosomcs from mycobacteria, nocardial and corynebacteria, and microorganisms in leprosy lesions. Infect. Immun. 22(1978 )540-547.
14. Phillips, I. A guide to sensitivity testing. J. Antimicrob. Chemother. 27 Suppl. D (1991)1-48.
15. Ridley, D. S. Histological classification and the immunological spectrum of leprosy. Bull. Who 51(1974)451-464.
16. Sneath, P. H. A., Main, N. S., Sharpe, M. E. and Holt, J. G. Bergey's Manual of Systemic Bacteriology Vol.2. Baltimore: Williams and Wilkins, 1986.
1. A. W. Sturm, M.D. Ph.D. Department of Microbiology, The Aga Kahn University, Karachi, Pakistan.
2. B. Jamil, M.B.B.S. Department of Microbiology, The Aga Kahn University, Karachi, Pakistan.
3. K. P. W. J. MacAdam, M.A. (Nat.Sc), M.R.C.P., F.R.C.P., London School of Hygiene and Tropical Medicine, London, U.K.
4. K. Z. Khan, M.Sc. D.Bact. Department of Microbiology, The Aga Kahn University, Karachi, Pakistan.
5. S. Parveen, B.Sc. M.Sc. Department of Microbiology, The Aga Kahn University, Karachi, Pakistan.
6. T. Chiang. M.B.B.S.. M.Sc, C.T.M. Marie Adelaide Leprosy Center, Karachi, Pakistan.
7. R. Hussain, B.Sc. (Hons), M.Sc, Ph.D., M.R.C. (Path), Department of Microbiology, The Aga Kahn University, Karachi, Pakistan.
Reprint requests to Prof. A. W. Sturm, Department of Microbiology, Medical School. University of Natal, P. O. Box 17039, Congella 4013, South Africa.
Received for publication on 24 October 1995.
Accepted for publication in revised form on 12 March 1996.