- Volume 64 , Number 3
- Page: 299–305
Recognition of lipid antigens by sera of mice infected with Mycobacterium lepraemurium
ABSTRACT
Lipids extracted f rom mouse tissues infected with Mycobacterium lepraemurium (MLM) were analyzed by thin-layer chromatography. Although the extracted lipids were heterogeneous in polarity, the lipids of intermediate polarity were the ones that predominated. All of the lipids of intermediate polarity were glycosylated species. There were also lipids of low and high polarity, the latter being glycolipids. Compared to lipids extracted f rom normal tissue (mostly lipids of high and low polarity), all of the additional lipids extracted f rom the infected tissue corresponded to lipids present in the purified bacteria. Enzyme-linked immunoassays (ELlSAs) were then performed with the whole lipids extracted f rom purified bacilli, the lipids of high, intermediate and low polarity, and the sera f rom 20 normal and 20 MLM-infected mice. Lipids of intermediate polarity were specifically recognized by MLM-infected mice. Neither sera (diluted 1:500) f rom normal mice nor infected mice reacted with the lipids of high or low polarity, but a higher concentration (sera diluted 1:100) of some sera f rom mice in both groups reacted significantly with these lipids. In the ELlSAs the whole-lipid extract and the lipids of intermediate polarity were similarly recognized by the sera of the infected mice. Thus, as observed in human leprosy, the mycobacterial disease in the mouse (murine leprosy) is also accompanied by the development of antibodies to the glycolipids of the infecting microorganism.
RÉSUMÉ
Les lipides extraits de tissus de souris infectées par Mycobacterium lepraemurium (MLM) ont été analysés par chromatographie en couche mince. Bien que les lipides extraits étaient hétérogènes du point de vue de lur polarité, les lipides de polarité intermédiaire étaient prédominants. Tous les lipides de polarité intermédiaire étaient de l'espèce glycosylée. Il y avait aussi des lipides de polarité faible et élevée, ces derniers étant des glycolipides. Comparés aux lipides extraits de tissus normaux (essentiellement des lipides de polarité élevée et basse), tous les lipides supplémentaires extraits des tissus infectés correspondaient aux lipides présents dans les bactéries purifiées. Des tests immuno-enzymatiques (ELISA) ont alors été utilisés avec les lipides entiers extraits des bacilles purifiés, les lipides de polarité élevée, intermédiaire et faible et les serums de 20 souris normales et 20 souris infectées par MLM. Les lipides de polarité intermédiaire étaient spécifiquement roconnus par les souris infectées par MLM. Ni les serums (dilution 1:500) de souris normales, ni ceux de souris infectées n'ont réagi avec les lipides de polarité élevée ou faible, mais une concentration plus élevée (1:100) de quelques serums de souris dans les deux groupes a réagi de manière significative avec ces lipides. Dans les ELISA, les extraits de lipides entiers et les lipides de polarité intermédiaire étaient également reconnus par les serums des souis infectées. Donc, comme on l'a observé dans la lèpre humaine, la maladie mycobactérienne chez la souris (lèpre murine) s'accompagne également du développement d'anticorps vis-à-fis des glycolipides des microorganismes infectants.RESUMEN
Se hizo el análisis por cromatografía en capa lina de los lípidos extraídos de tejidos de ratones infectados con Mycobacterium lepraemurium (MLM). Aunque los lípidos extraídos fueron heterogéneos en polaridad, los lípidos predominantes fueron los de polaridad intermedia. Todos los lípidos de polaridad intermedia fueron especies glicosiladas. También hubieron lípidos de baja y de alta polaridad, estos últimos también fueron glicolípidos. Comparados con los lípidos extraídos del tejido normal (en su mayoría lípidos de alta y de baja polaridad), todos los lípidos adicionales extraídos de los tejidos infectados correspondieron a lípidos presentes en la bacteria purificada. Con estas familias de lípidos se hicieron inmunoensayos enzimáticos (ELlSAs) utilizando los sueros de 20 animales infectados con MLM y de 20 animales normales. Los lípidos de polaridad intermédia fueron especificamente reconocidos por los ratones infectadoes con MLM. Ni los sueros de los ratones normales (diluidos 1:500) ni los sueros de los animales infectados reaccionaron con los lípidos de alta o de baja polaridad. Sin embargo, a concentraciones mayores de los sueros (sueros diluidos 1:100) algunos de los ratones de ambos grupos reaccionaron significativamente con estos lípidos. En los ELlSAs el extracto lipídico total y los lípidos de polaridad intermédia fueron reconocidos de manera similar por los sueros de los ratones infectados. Así, como ocurre en la lepra humana, la lepra murina también se acompana por el desarrollo de anticuerpos contra los glicolípidos dei microorganismo infectante.Mycobacterial lipids and glycolipids are an important group of macromolecules due to their peculiar chemical characteristics and properties (3,12). Lipids very likely participate in the resistance of pathogenic mycobacteria to the deleterious action of the host's phagocytes (8,13,14,29), and they may function as virulence factors (5,27). Lipids have been shown to be the target structures for humoral (4,16,19) and cellular immunity (9,11). Lipids are very likely responsible for much of the immunopathology of mycobacterial diseases. So far, the bulk of information on the chemical and biological characteristics of mycobacteria has been derived from the study of species that are pathogenic to man, such as Mycobacterium leprae, M. tuberculosis and its relative BCG, not to mention M. avium that has gained much attention due to its association with AIDS. There are, however, other mycobacterial species, nonpathogenic to man, such as M. microti and M. lepraemurium (MLM), of similar biological importance. These are special cases since they are pathogenic to mice, the most popular laboratory animal for biological research. Murine leprosy, on the other hand, resembles human leprosy in many aspects, including those related to the immunology and histopathology of the disease.
Our laboratory has taken murine leprosy as a working model to understand the general immunopathogenic mechanisms of chronic mycobacterial diseases. In this paper we report the profile of the lipids extracted from infected tissue and from purified bacilli and their immunological recog-nition by sera of mice infected with MLM.
MATERIALS AND METHODS
Source of bacteria and lipids. M. lepraemurium, Hawaiian strain, was grown in female, albino NIH mice. Infected tissues (liver, spleen and lepromas) were routinely harvested 4 to 6 months after the intraperitoneal injection of mice with 107 to 108 bacilli. Liver and spleen from normal animals and M. lepraemurium purified by Prabhakaran's method (21) served as the sources for the control lipids. An extra source of lipids was a subcutaneous leproma taken from a nine-banded armadillo (Dasypus novemcinctus) heavily infected with M. leprae.
Lipid extraction. Lipid extraction was performed according to Folch, et al. (10), with some modifications. Briefly, per g of intact murine or armadillo-infected tissue, 4 ml of physiological saline was added and the tissue was homogenized in a tissue blender. The volume of the resulting suspension was measured; to each 2 ml of tissue suspension or purified MLM suspension, 7.5 ml of a chloroform: methanol (1:2) mixture was added and the suspension was then incubated overnight at 50 ºC in a screw-cap container. The suspension was then filtered, the filtrate mixed with 10 ml of chloroform: water (1:1), and the mixture transferred to a separation funnel. After separation of phases, the organic phase was collected and dried in a rotavapor.
Chromatographic analysis. The analysis of lipids was performed by thin-layer chromatography (TLC) on aluminium sheets with silica gel 60 F254 (Merck & Co., Inc., Rahway, New Jersey, U.S.A.). The solvent systems used were: a) hexane: ether:acetic acid (80:20:1) for low polarity lipids; b) chloroform : methanol (100:10 and 100:15) for lipids of intermediate polarity; c) chloroform : methanol: water (100:30:3) for high-polarity lipids. To develop, TLC plates were exposed to iodine vapors (low-polarity lipids; LPL) or sprayed with 0.1 % orcinol in 60% H2SO4 [lipids of intermediate (1PL) and high'(HPL) polarity] and heated. Unless otherwise specified, chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, Missouri, U.S.A.).
Preparation of antigens for ELISAs. The lipid antigens were prepared by chromatography on columns of silica gel 60 (Merck). About 1 g of the lipids extracted from MLM-infected tissue was layered on a 20 x 150 mm column of silica gel previously washed and equilibrated with chloroform. Sequential elution with chloroform, acetone and methanol, allowed us to separate families of lipids of different polarities. The volume of each solvent used was variable and dependent upon the separation of the lipid fractions (visually assessed on the basis of color or appearance). Each family of lipids was collected and dried in a rotavapor. For analysis by TLC, samples of the lipids were taken, weighed, and dissolved in chloroform at the concentration of 1 mg per ml, they were then applied on the TLC sheets at comparable concentrations (as assessed by observation of the folio under ultraviolet light). Chromatograms were run in a solvent mixture of chloroform: methanol (100:8). They were then air dried, sprayed with orcinol-ferric chloride (Bial's reagent), air dried, sprayed with 5% sulfuric acid in ethanol, and heated in an oven until the desired contrast of colors was reached.
Enzyme-linked immunoassay (ELISA). For ELISA, 1.0 mg of each lipid was first dissolved in 0.1 ml of chloroform and then diluted to 10 ml with absolute ethanol (100 µg per ml). One-hundred µ l of lipid solutions (10 µg) were added per well of Nunc micro-ELISA plates (Nunc, Roskilde, Denmark); control wells were treated only with ethanol. After overnight incubation of the plates at 37ºC to allow solvent evaporation, the wells were washed once with borate-buffer solution (BBS) (0.3092 g boric acid and 0.4768 g sodium tetraborate per liter of 0.85% sodium chloride, pH 8.4), incubated for 30 min with 0.15 ml of 2% defatted milk (DM; Nestle) in BBS, incubated for 30 min at 37ºC, and then incubated for 3 hr with the test sera diluted 1:100 or 1:500 in 2% DM made up in phosphate buffer solution (PBS) (0.01 N phosphate, 0.15 N sodium chloride, pH 7.4). Wells were then washed four times with 150 µ l of PBS and then incubated with 100 µ l of a mixture of peroxidase-linked anti-mouse IgG and IgM prepared at an optimal predetermined dilution in 2% DM in PBS (see Results). After incubation for 60 min at 37ºC, the excess reagents were washed out with PBS as before, and then 100 µ l of the chromogensubstrate mixture (4 mg ortho-phenylenediamine and 10 µ l of hydrogen peroxide in 10 ml of 0.5 M acetate buffer, pH 5.0) were added per well. Twenty min later the reaction was stopped with 4 NH4S04 (50 µ l per well, and the absorbances were read at 492 nm. Blank wells for antigen and antibody were included and used to correct the readings.
Statistical analysis. The results were analyzed by the Student's t test for small samples.
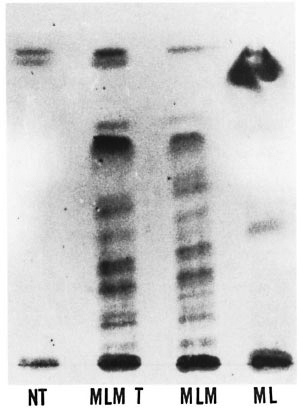
Fig . 1. Thin-layer chromatography of lipids ex-tracted from normal tissue (NT), Mycobacterium lepraemurium -infected tissue (MLMT), purified M. leprraemurium (MLM) and purified M. leprae (NIL) [Silica gel 60 F254, Merck; chloroform-methanol (100:8);oreinol-sulfuric acid stain].
RESULTS
Analysis of lipids. The analysis of lipids by TLC revealed important differences between the lipids extracted from normal and MLM-infected tissue. Extracts from normal tissue (spleen and liver) were almost devoid of lipids of intermediate polarity. In contrast, MLM-infected tissue showed additional lipids of intermediate and high polarity that reacted with orcinol and alpha-naphthol, indicating that they are glycolipids. The TLC pattern of these additional lipids was similar to the pattern of the lipids extracted from purified M. lepraemurium. Compared to the murine situation, the lipid extract prepared from the M. leprae -infected (armadillo) tissue contained less lipids of intermediate polarity and more lipids of low and high polarity (Fig. 1).
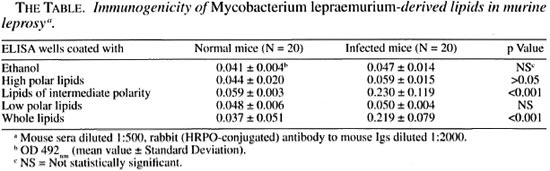
Serology. On the basis of their differential polarity, the lipids extracted from the infected tissue were separated into three groups of lipid families (Fig. 2). These lipids and the ones extracted from purified MLM bacilli were tested by ELISA against 20 sera from normal mice and 20 sera from mice with > 4 months of infection with MLM. In the experiment summarized in The Table, with the sera diluted 1:500, while the lipids of intermediate polarity extracted from infected tissue reacted in a specific manner with the sera from MLM-infected mice (OD492 nm = 0.230 ± 0.119 vs. 0.059 ± 0.003 with normal sera, p = 0.001), the lipids of low and high polarity were not significantly recognized by the sera of either normal or infected animals. Lipids from purified MLM bacilli were also specifically recognized by the sera of infected mice (OD = 0.219 ± 0.079 vs. 0.037 ± 0.051 with normal sera, p = 0.001; The Table). Lower dilutions of the sera (1:100) gave higher readings, but the overall results remained basically the same even when some reactivity between some normal or infected sera and high- or low-polar lipids was also observed (Fig. 3).
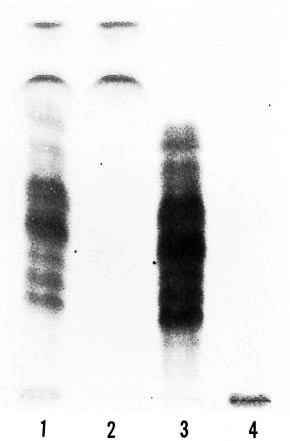
Fig 2. Thin-layer chromatography of lipids ex-tracted from MLM-infected tissue. Whole lipids (laneI) were further separated into low polar lipids, LPL(lane 2); lipids of intermediate polarity. IPL (lane 3);high polar lipids, HPL. (lane 4). Each lipid family wasthen used as antigen in the EL1SAs [Silica gel 60F254, Merck; chloroltirm-methanol ( 100:8); orcinol-sulfuric acid stain.]
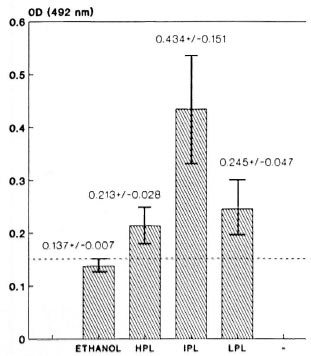
Fig. 3. Immunological recognition of M. leprae-murium -derived lipids by the sera from 20 MLM-in-fected animals. Lipids of high (11PL), intermediate(IPL), and low (LPL) polarity were used as antigens atconcentration of 10 µ g per well; sera were used diluted1:100. Average reading of sera tested on ethanol-coated wells (no antigen) is also shown. Bars are the mean values (± one Standard Deviation) of three identical experiments with sera tested in triplicate.
The average reading of the sera tested on ethanol-coated wells (no antigen) is also shown in Figure 3. In this experiment with mouse leprosy sera, the average readings corrected by subtracting the "ethanol reading" are 0.076 for HPL, 0.297 for IPL, and 0.108 for LPL. Once again, the mycobacterial lipids of intermediate polarity are the ones more intensely recognized by the sera of mice infected with MLM.
DISCUSSION
The bulk of information on humoral responses developed by infected hosts against mycobacterial lipid antigens is derived from the study of the diseases caused by M. leprae, M. tuberculosis and the M. avium complex (MAC) (2,3,12). In all instances, the mycobacterial lipids of intermediate polarity have been shown to be immunogenic, both under natural and experimental conditions of disease (6,17,18,20,28). Some of these lipid antigens have been proposed as tools for the diagnosis and follow up of leprosy (phenolic glycolipid-I) (1,22) , tuberculosis (acyltrehaloses and phenolic glycolipid Tb)(7,23,26), and diseases caused by representatives of the MAC (5-mycoloyl-β-arabinofuranosyl-1 (1 -2)-5-mycoloyl-α-arabinofuranosyl glycerol) (15,28). In our study we found that mice infected with MLM produced antibodies to mycobacterial lipids of high and intermediate polarity, however, only the lipids of intermediate polarity were recognized by the sera of infected mice but not by the sera of normal mice.
On the other hand, the lipid pattern of MLM was more complex than that of M. leprae; MLM does not contain phenolic glycolipid-I (PGL-I) nor any other predominant lipid species. The existence of a variety of mycobacterial lipids in the lesions of murine leprosy has been previously reported by our group (24) and by Sakura and Skinsnes (25). Glycolipids, phospholipids and neutral fats (in decreasing order) were the predominant lipids found; no sulfatides or cholesterol were detected.
In the present study, the chromatographic analysis of the different lipid families allowed us to identify those lipids derived from the bacteria that were immunogenic in the mouse. At a low concentration of the sera (sera diluted 1:500), only the lipids of intermediate polarity were specifically recognized by the infected mice. Sera from normal mice were unreactive to lipids of intermediate polarity. Low-polar and high-polar lipids were in general nonimmunogenic in mice infected with MLM and were not recognized by the sera of normal mice. Moderate recognition of high- or low-polar lipids by the sera diluted 1:100 was observed in some normal and MLM-infected mice. This could be the result of either a nonspecific chemical reaction or the cross-reactivity with lipids of environmental microorganisms not necessarily related to mycobacteria. The mixture of lipids extracted from purified MLM bacilli were recognized by the sera of infected mice, but the observed reaction was not higher than the one seen with only the group of lipids of intermediate polarity; this underlines the predominant antigenicity of this latter type of lipids. In summary, in this report on murine leprosy we present evidence of the immunogenicity of MLM lipids under the pathophysiologic conditions of the disease, a situation similar to the one observed in human leprosy with regard to the antigenicity of the lipids of M. leprae.
Acknowledgment. This work received financial support from the Dirección de Estúdios de Posgrado e Investigación dei IPN, México (Proyectos DEPI 880698, 892408, and 952281 "Lípidos micobacterianos en la lepra murina experimental"). Authors wish to thank Dis. Juvencio Ruiz Puente and Ana Elena Cirandes Robles, from the Instituto Nacional de Higiene, for their gênerons periódica] supply of specific pathogen-tree NIH mice. J. Luna-Herrera was a fellow of COSNET and COTEPABE, S. Estrada-Parra and O. Rojas-Espinosa are fellow-holders of COFAA (IPN), EDD (IPN) and SNI, México.
REFERENCES
1. Bach, M. A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid I and to whole Mycobacterium leprae in leprosy patients: evolution and therapy. Int. J. Lepr. 54(1986)256-267.
2. Brennan, P. J. Antigenic peptidoglycolipids, phospholipids and glycolipids. In: The Mycobacteria; a Source Hook. Kubica, G. P. and Wayne, L. G., eds. New York/Basel: Marcel Dekker, Inc., 1984, pp. 467-490.
3. Brennan, P. J. Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev. Infect. Dis. 11(1989)s420s430.
4. Brennan, P. J. and Goren, M. B. Structural studies on the type-specific antigens and lipids of Mycobacterium avium - Mycobacterium intracellular - Mycobacterium scrofulaceum serocomplex. J. Biol. Chem. 254(1979)4205-4211.
5. Chan, J., Fan, X., Hunter, S. W., Brennan, P. J. and Bloom, B. R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 59(1991)1755-1761.
6. Cho, S., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
7. Cruaud, P., Yamashita, J. T., Casabona, N. M., Papa, P. and David, H. L. Evaluation of a novel 2,3-diacyl-trehalose-2'-sulfate (SL-IV) antigen for case finding and diagnosis of leprosy and tuberculosis. Res. Microbiol. 141(1990)679-694.
8. De Chastellier, C, Frehel, C, Offredo, C. and Skamene, E. Implication of phagosome-lysosome fusion in restriction of Mycobacterium avium growth in bone marrow macrophages from genetically resistant mice. Infect. Immun. 61(1993)3775-3784.
9. Ellner, J. J. and Daniel, T. M. Immunosuppression by mycobacterial arabinomannan. Clin. Exp. Immunol. 35(1979)250-257.
10. Folch, J.. Lees. M. and Sloane, S. G. H. A simple method for the isolation of total lipids from animal tissues. J. Biol. Chem. 226(1957)497-509.
11. Fournie, J. J., Adams, E.. Mullins, R. J. and Basten, A. Inhibition of human proliferative responses by mycobacterial phenolic glycolipids. Infect. Immun. 57(1989)3653-3659.
12. Goren, M. B. and Brennan, P. J. Mycobacterial lipids: chemistry and biological activities. In: Tuberculosis. Youmans, G. P., ed. Philadelphia: W. B. Saunders Co., 1979. pp. 63-193.
13. Gorhn. M. B., D'Arcy Hart. P., Young, M. R. and Armstrong, J. A. Prevention of phagosomelysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc. Nat. Acad. Sci. U.S.A. 73(1976)510-2514.
14. Hines, M. E., Jaynes, J. M., Barker, S. A., Newton, J. C, Enright, F. M. and Snider, T. G. Isolation and partial characterization of glycolipid fractions from Mycobacterium avium serovar 2 (M. paratuberculosis 18) that inhibit activated macrophages. Infect. Immun. 61(1993)1-7.
15. Honda, I., Kawajiri, K., Watanabe, M., Toida, I., Kawamata, K. and Minnikin, D. E. Evaluation of the use of 5-mycoloyl-β-arabinofuranosyl-l (I -2)5-mycoloyl-α-arabinofuranosyl-(I-l')-glycerol in serodiagnosis of Mycobacterium avium-intracellulare complex infection. Res. Microbiol. 144(1993)229-235.
16. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
17. Hunter, S. W., Fujiwara, T, and Brennan, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
18. Minnikin, D. E., Ridell, M., Parlett, J. H. and Bolton, R. C. Direct detection of Mycobacterium tuberculosis lipid antigens by thin layer chromatography. FEMS Microbiol. Lett. 48(1987)175-177.
19. Papa, F. Craud, P. and David, H. L. Antigenicity and specificity of selected glycolipid fractions from Mycobacterium tuberculosis. Res. Microbiol. 1401989)569-578.
20. Papa, E, Laszlo, A. and David, H. L. Specificity of Mycobacterium tuberculosis phenolic glycolipid (PGL-Tb-I) antiserum. Ann. Inst. Pasteur/ Microbiol. Lett. 139(1988)535-545.
21. Prabhakaran, K., Harris, E. B. and Kirchheimer, W. F. Binding of 14C-labeled Dopa by Mycobacterium leprae in vivo. Int. J. Lepr. 44(1976)58-64.
22. Prakash, K., Aggarwal, R. and Sehgal, V. N. Significance of antibodies to phenolic glycolipid-I in leprosy diagnosis. J. Dermatol. 19(1992)953-958.
23. Ridell, M., Walllerstrom, G., Minnikin, D. E., Bolton, R. C. and Magnusson, M. A comparative serological study of antigenic glycolipids from Mycobacterium tuberculosis. Tuberc. Lung Dis. 73(1992)101-105.
24. Rojas-Espinosa, O. anil Maldonado-Reyes, E. Cutaneous lipids and mast cells in murine leprosy. Int. J. Lepr. 59(1991)325-331.
25. Sakura, I. and Skinsnes, O. K. Lipids in leprosy. 1. Histochemistry of lipids of murine leprosy. Int. J. Lepr. 38(1970)379-388.
26. Torgal-Garcia, J., David, H. L. and PAPA, F. Preliminary evaluation of a Mycobacterium tuberculosis phenolic glycolipid antigen in the serologic diagnosis of tuberculosis. Ann. Inst. Pasteur 139(1988)289-294.
27. Vachula, M, Worobec, S. and Andersen, B. R. A comparison of monocyte oxidative responses in leprosy patients and healthy subjects as influenced by mycobacterial lipid pretreatment. Int. J. Lepr. 58(1990)534-539.
28. Watanabe, M., Kudoh, S., Yamada, Y., Iguchi, K. and Minnikin, D. E. A new glycolipid from Mycobacterium avium-Mycobacterium intracellulare complex. Biochem. Biophys. Acta 1165(1992)53-60.
29. Zhang, L., Goren, M. B., Holzer, T. J. and Andersen, B. R. Effect of Mycobacterium tuberculo sis-derived sulfolipid-1 on human phagocytic cells. Infect. Immun. 56(1988)2876-2883.
1. Sc.D. Department of Immunology, National School of Biological Sciences, National Polytechnic Institute, Carpió y Plan de Ayala, Colonia Santo Tomas, 11340 Mexico, D.F., Mexico.
2. Sc.D.Department of Immunology, National School of Biological Sciences, National Polytechnic Institute, Carpió y Plan de Ayala, Colonia Santo Tomas, 11340 Mexico, D.F., Mexico.
3. Ph.D., Department of Immunology, National School of Biological Sciences, National Polytechnic Institute, Carpió y Plan de Ayala, Colonia Santo Tomas, 11340 Mexico, D.F., Mexico.
Reprint requests to Dr. Rojas-Espinosa.
Received for publication on 24 May 1995.
Accepted for publication in revised form on 1 February 1996.