- Volume 64 , Number 3
- Page: 253–6
Primary dapsone resistance in cebu, the philippines; cause for concern
ABSTRACT
At a time when primary dapsone resistance was prevalent in many leprosy-endemic areas, Cebu in The Philippines reported only 3.6% in the period 1975-1978 and later 8.1% in the period 1979-1982. In our current study of patients in the period 1988-1992, the number increased dramatically to 52.7%. In addition, 7.9% of the isolates are highly resistant to dapsone, a level of resistance not seen in earlier studies.This finding could have severe ramifications to the World Health Organization's multidrug therapy (WHO-MDT) mode of treatment, where dapsone is one of the principal drugs. Moreover, the increase in primary dapsone resistance may be a contributing factor in the recent finding that there has been no decline in the number of new cases found in Cebu, even after the implementation of WHO-MDT in 1985. There is a need for new drugs that could be included in the multidrug treatment for multibacillary and paucibacillary leprosy.
RÉSUMÉ
A une époque où la résistance primaire à la dapsone était présente dans beaucoup de régions où la lèpre était endémique, on ne rapportait à Cebu, dans les Philippines, que 3.6% de résistance primaire pour la période 1975-1978 et 8.1% pour la période 1979 - 1982. Dans notre étude actuelle des patients pour la période 1988-1992, cette proportion a augmenté de manière considérable jusqu'à 52.7%. De plus, 7.9% des isolats sont hautement résistants à la dapsone, et ce niveau de résistance n'avait pas été observé dans les études précédentes. Cette observations pourraient avoir plusieurs conséquences pour le mode de traitement par polychimiothérapie de l'Organisation Mondiale de la Santé (PCT-OMS), dans lequel la dapsone est l'un des médicaments principaux. De plus, l'augmentation de la résistance primaire à la dapsone pourrait être un facteur contributif à la découverte recent qu'il n'y a pas eu de diminution dans le nombre de nouveaux cas détectés à Cebu, même après l'introduction de la PCT-OMS en 1985. Il existe un besoin de nouveaux médicaments qui pourraient être inclus dans la polychimiothérapie pour la lèpre multibacillaire et paucibacillaire.RESUMEN
En la época en que la resistência primaria a la dapsona era prevalente en muchas áreas con lepra endémica, Cebu en las Filipinas reporto sólo un 3.6% de aislados resistentes a la droga en el periodo de 1979 a 19X2. En el presente estúdio que abarca el periodo de 1988 a 1992, encontramos que el número de aislados resistentes aumento dramaticamente a 52.7%. El 7.9% de los aislados lue altamente resistente a la dapsona, un nivel de resistência nunca visto en los estúdios anteriores. Ests hallazgo podría tenergran repercusión en el esquema de tratamiento con poliquimioterapia de la Organization Mundial de la Salud (PQT-OMS) donde la dapsona es una de las drogas principales. Adernas, el incremento en la resistência primaria a la dapsona podría ser un factor contribuyente al hallazgo reciente de que no ha habido una disniinución en el número de casos encontrados en Cebu aún después de haberse implementado la PQT-OMS en 1985. Es claro que se necesita la inclusion de nuevas drogas en la PQT para el tratamiento de la lepra multibacilar y paucibacilar.Primary dapsone (DDS) resistance is very prevalent in many leprosy-endemic areas of the world and represents a serious potential threat to the leprosy control programs in these regions (2,3) . Although virtually nonexistent before 1977 (11), numerous studies have shown that more than one third of newly diagnosed multibacillary (MB) patients in most leprosy-endemic areas are infected with DDS-resistant Mycobacterium leprae (7). One notable exception has been in Cebu, The Philippines. In 1981, Guinto, et al. reported primary DDS resistance in only 3.6% of patients studied from 1975 to 1978 (4). In a later study, Cellona, et al. reported primary DDS resistance in 8.1% of newly diagnosed patients studied in the period 1979 to 1982 (1).
The present study was conducted to determine the DDS sensitivity of isolates from new, previously untreated leprosy patients during the period 1988 to 1992 in Cebu, and to compare these results with those from the earlier studies. Our results demonstrate that primary DDS resistance has dramatically increased in Cebu, and the rate is much higher than in earlier studies.
MATERIALS AND METHODS
Biopsy specimens were obtained from 38 new, untreated borderline lepromatous/lepromatous (BL/LL) leprosy patients consecutively admitted for other studies during the period 1988 to 1992. The samples consisted of 8-mm punch biopsy specimens taken from active lesions with a skin smear of > 4+.
The tissue specimens were processed using the standard Shepard technique (9). Acid-fast bacilli (AFB) were recovered, stained by the Ziehl-Neelsen method and counted (10). Suspensions of 5 x 103 bacilli were injected into each hind foot pad of inbred CBA/J mice; 40 to 60 mice were inoculated per specimen and divided into four groups. The control group was fed untreated, pelleted mouse chow. The three experimental groups received diets mixed with dapsone in concentrations of 0.0001,
0.001 and 0.01 g per 100 g of powdered diet. The chow was administered continually from day 1 until the mice were sacrificed. All inoculated mice were housed in temperature-controlled rooms maintained at 68°F-72°F. Mice were killed by cerebral dislocation. Harvesting organisms from the foot pads was done 12 months after inoculation. Both hind foot pads of individual mice were pooled, processed, stained and counted. A harvest of 1 x 105 AFB per foot pad, or a 20-fold increase in the count of the inoculum, was considered a positive harvest.
RESULTS
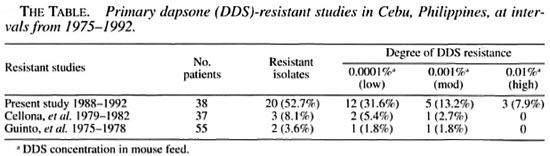
The results of the present study (1988 - 1992) are summarized in The Table. Of the 38 patients tested, 20 patients (52.7%) were infected with DDS-resistant M. leprae. In those patients with resistance, 31.6% were infected with low resistant M. leprae, 13.2% were infected with moderately resistant M. leprae and 7.9% were infected with leprosy bacilli highly resistant to DDS. The resultsofthis study are in sharp contrast to two earlier studies in 1975-1978 (Guinto, et al.1) and in 1979-1982 (Cellona, et al.') in which only 3.6% and 8.1%ofpatients, respectively, were infected with DDS-resistant M. leprae. Moreover, in these earlier studies the M. leprae isolates were either low or moderately resistant to DDS; none were highly resistant to DDS. Although in the 1979-1982 study, the rate of resistance had more than doubled compared to the 1975-1978 study (8.1% vs. 3.6%), the percentages were low and not viewed as alarming. The isolates were processed using the same methods in all three studies.
DISCUSSION
Dapsone is still one of the most important of the antilcprosy drugs used today. It is stable, inexpensive, relatively nontoxic and one of the mainstays of the World Health Organization multidrug therapy (WHOMDT) for both MB and paucibacillary (PB) leprosy. Unfortunately, developmentofacquired or secondary resistance in patients treated with DDS monotherapy for many years has provided a sourceofinfection resulting in the appearanceofprimary DDS resistance among new patients diagnosed with the disease. It is reported that primary DDS resistance is not confined only to patients with BL/LL leprosy, but to all types of leprosy, although it is difficult to prove resistance in patients with low bacterial indexes (8).
The results of this study suggest that more than half of all new patients in Cebu could be infected with DDS-resistant M. leprae. In addition, M. leprae isolates highly resistant to DDS were seen in patients in the most recent study - a level of resistance not found in the earlier studies. It is noteworthy that the DDS concentration in mouse chow testing for high resistance to DDS (i.e., 0.01%) is equivalent to a daily dose of 100 mg of DDS in humans. On the other hand, most of the resistant cases are of low (31.6%) or moderate resistance (13.2%). DDS will, therefore, still exert some effect on the majority of the new patients except, of course, on the smaller percentage of patients infected with M. leprae highly resistant to DDS.
While the majority of resistant strains of M.leprae in Cebu are as yet of a low level, it is generally believed that the mutation which produces DDS resistance develops progressively in a "step wise" manner rather than as a "single step" process, implying an evolutionary element in the development of higher degrees of resistance (5). Our resistance studies in the mouse foot pad in Cebu clearly demonstrate this progressive nature in the development of resistance. In a span of a decade or more, there has been an increase not only in the total number of patients developing primary DDS resistance, but also a progressive increase in the number of patients developing higher levels of resistance. Thus, there is the potential that eventually a large number of new patients in Cebu could be infected with M. leprae highly resistant to DDS.
A population of new patients infected with leprosy bacilli highly resistant to DDS has very serious ramifications for patients given WHO-MDT. The WHO-MDT regimen for MB patients involves three drugs (rifampin, clofazimine and dapsone) but a significant number of patients avoid clofazimine because of the darkening effect upon the skin. Such patients would theoretically end up receiving rifampin monotherapy to which resistance will develop (6). A similar situation could develop in MB patients wrongly diagnosed as PB patients in the field, leaving them with only rifampin given monthly for 6 months as their effective medication. This problem is further compounded in Cebu by the recent finding that there has been no decline in the number of new cases of leprosy since WHO-MDT was implemented a decade ago in 1985 (Balagon, et al., submitted for publication). However, it is not known at this time if DDS resistance has been a contributing factor in the lack of decline in new cases in Cebu. Further studies may clarify this scenario.
CONCLUSIONS
There is a dramatic increase in primary DDS resistance in Cebu. More than 50% of untreated, newly diagnosed patients are harboring resistant M. leprae and nearly 8% of these isolates are highly resistant to DDS. The incidence of leprosy in Cebu has not declined in spite of a decade of WHOMDT, emphasizing the need for new drugs that could be included in the multidrug treatment for MB and PB leprosy.
Acknowledgment. We are very grateful to American Leprosy Missions, Greenville, South Carolina, U.S.A.; Sasakawa Memorial Health Foundation, Tokyo, Japan and the Damien Dutton Society, Bell-more, New York, U.S.A. for providing financial support for the research programs at the Leonard Wood Memorial (LWM) Center in Cebu. We are also grateful to Mrs. Salud Guinto for her encouraging support of the LWM in Cebu. We also gratefully acknowledge the technical assistance of Mrs. Rhea M. Fajardo. Mrs. Paulina S. Munalem, Ms. Manuela Luisa Parrilla and Mr. Junie F. Abellana. Finally, our thanks to Mr. Bernardo P. Mendo/a and Mr. Rico P. Abella for the clerical and computer work on the manuscript
REFERENCES
1. Cellona, R. V., Fajardo, T. T, Jr., Kim, D. I., Hah, Y. M., Ramasoota, T., Sampattavanich, S., Carrillo, M. P., Abalos, R. M., Dela Cruz, E. C, Ito, T. and Yuasa, Y. Joint chemotherapy trials in lepromatous leprosy conducted in Thailand, The Philippines and Korea. Int. J. Lepr. 58(1990)1-11.
2. Chen, J., Wang. S., Haw, Y. Ni, G., Zhang, J. and Tang, Q. Primary dapsone resistance in China. Lepr. Rev. 60(1989)263-266.
3. Guelpa-Lauras, C. C, Cartel, J. L., ConstantDesportes, M.. Millan, J., Bobin, P., Guidi, C, Brucker, G., Flageul, B., Guillaume, J. C, Picket, C, Remy, J. C. and Grosset, J. H. Primary and secondary dapsone resistanceof M. leprae in Martinique, Guadaloupe, New Caledonia, Tahiti, Senegal and Paris between 1980 and 1985. Int. J. Lepr. 55 (1987)672-679.
4. Guinto, R. S., Cellona, R. V., Fajardo, T. T. JR. and Dela Cruz, E. C. Primary dapsone-resistant leprosy in Cebu, Philippines. Int. J. Lepr. 41(1981)427-430.
5. Hastings, R. C. Growthofsulfone-resistant M. leprae in the foot padsofmice fed with dapsone. Proc. Soc. Exptl. Biol. Med. 156 (1977)544-545.
6. Jacobson, R. R. and Hastings, R. C. Rifampin resistant leprosy. Lancet 2(1976)1304-1305.
7. Jt, B. Drug resistance in leprosy - a review. Lepr. Rev. 56 (1985)265-278.
8. Jt, B. and Grosset, J. H. Recent advances in the chemotherapy of leprosy - an editorial. Lepr. Rev. 61(1990)313-329.
9. Shepard, C. C. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112 (I960)445-454.
10. Shepard, C. C. and McRae, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36(1968)78-82.
11. Shepard. C. C, Rees, R. J. W. Levy, L., Pattyn, S. R., Jt, B. and Dela Cruz, E. C. Susceptibility of strainsof Mycobacterium leprae isolated prior to 1977 from patients with previously untreated lepromatous leprosy. Int. J. Lepr. 54(1986)11-15.
1. D.V.M., Chief, Vivarium Department;
2. M.D., D.P.H. (Lond.), Chief, Epidemiology Branch;
3. M.D., Clinical Assistant, Cebu Skin Clinic;
4. M.D., Chief, Clinical Branch;
5. M.D., M.P.H., Senior Clinical Consultant;
6. M.D., Pathologist;
7. M.D., Immunologist;
8. Ph.D., Director, Leonard Wood Memorial, P.O. Box 727, Cebu City 6000, The Philippines.
Received for publication on II December 1995.
Accepted for publication in revised form on 12 March 1996.