- Volume 64 , Number 3
- Page: 257–67
Restoration of proliferative response to M. leprae antigens in lepromatous t cells against candidate antileprosy vaccines
ABSTRACT
Several studies conducted in the last decade suggest that Mycobacterium leprae- reactive T cells exist in lepromatous patients, but their number may be too few to yield a detectable response in cell-mediated immunity (CMI) assays. Immunizations with candidate antileprosy vaccines and stimulation of T cells with M. leprae + interleukin-2 restore the M. leprae -induced CMI response in lepromatous leprosy patients. These immunizations and stimulation may enrich the pre-existing M. leprae- responsive T cells in lepromatous patients and, thereby, induce a detectable CMI response to M. leprae antigens upon repeat testing. To verify this proposition, we carried out a study in a group of 10 lepromatous leprosy patients. Peripheral blood mononuclear cells (PBMC) obtained f rom these patients were anergic to M. leprae antigens in proliferative assays, but they responded to the antigens of candidate antileprosy vaccines, i.e., M. bovis BCG, M. bovis BCG + M. leprae, and Mycobacterium w. The enrichment of M. leprae-re sponsive T cells was performed by establishing T-cell lines f rom the PBMC after in vitro stimulation with M. leprae, M. bovis BCG, M. bovis BCG + M. leprae, and Mycobacterium w. When tested for their proliferative responses, 1/10, 3/10, 6/10 and 2/10 T-cell lines established against M. leprae, M. bovis BCG, M. bovis BCG + M. leprae, and Mycobacterium w, respectively, responded to M. leprae. These results suggest that enrichment of pre-existing M. leprae responsive T cells may contribute to the restoration of the T-cell response to M. leprae in some lepromatous patients. Four of the 10 M. leprae -induced T-cell lines proliferated in response to the 65 kDa, 36 kDa, 28 kDa, and 12 kDa recombinant antigens of M. leprae, suggesting that the nonresponsiveness of T cells in some lepromatous patients may be overcome by using recombinant antigens of M. leprae.RÉSUMÉ
Différentes études réalisées au cours de la denière décennie suggèrent que des cellules T réactives à Mycobacterium leprae existent chez, les malades lépromateux, mais leur nombre pourrait être trop faible pour provoquer une réponse détectable par les tests de l'immunité à médiation cellulaire (IMC). Des immunisations avec des candidats vaccins anti-lèpre et la stimulation des cullules T par M. leprae + interleukine-2 restaurent la réponce d'IMC induite par M. leprae chez, les lépreux lépromateux. Ces immunisations et la stimulation peuvent enrichir des cellules T réactives à M. leprae pré-existantes chez, des malades lépromateux, et, par là, provoquer ine réponse IMC détectable vis-àvis des antigènes de M. leprae lors de tests à répétition. Pour vérifier cette hypothèse, nous avons réalisé une étude dans un groupe de dix malades lépromateux. Les cellules mononucléaires du sang périphérique (CMSP) obtenues de ces patients étaient anergiques aux antigènes de M. leprae dans les tests de prolifération, mais répondaient aux antigènes de candidats vaccins anti-lèpre BCG de M. bovis, BCG de M. bovis + M. leprae, et Mycobacterium w. L'enrichissement des cellules T réactives à M. leprae a été réalisé en établissant des lignées de cellules T à partir de CMSP après stimulation in vitro par du M. leprae, BCG de M. bovis, BCG de M. bovis + M. leprae, et Mycobacterium w. Quand on les a testées pour leurs réponses proliférâmes, respectivement 1/10, 3/10, 6/10 et 2/10 des lignées de cellules T établies contre M. leprae, BCG de M. bovis, BCG de M. bovis + M. leprae, et Mycobacterium w ont répondu à M. leprae. Ces résultats suggèrent que l'enrichissement de cellules T pré-existantes réactives à M. leprae pourraient contribuer à la restauration de la réponse des cellules T vis-à-vis de M. leprae chez, certains malades lépromateux. Quatre des 10 lignées de cellules T induites par M. leprae ont proliféré en réponse aux antigènes recombinants de 65 kDa, 36 kDa, 28 kDa et 12 kDa; ceci suggère que la non-réactivité des cellules T chez certains malades lépromateux pourrait être vaincue par l'utilisation d'antigènes recombinants de M. leprae.RESUMEN
En la última década se han realizado varios estudios que sugieren que en los pacientes con lepra lepromatosa existen células reactivas con Mycobacterium leprae pero que su número es tan pequeño que es difícil detectarlas en los ensayos de immunidad celular. La inmunización con vacunas potenciales contra la lepra y la estimulación de las células T con M. leprae + interleucina 2, restauran la respuesta de inmunidad celular en los pacientes lepromatosos. Las inmunizaciones y la estimulación podrían enriquecer la población preexistente de células reactivas con M. leprae facilitando así la delección de las respuestas celulares hacia los antígenos del microorganismo. Para verificar esta suposición se hizo un estudio en un grupo de 10 pacientes con lepra lepromatosa. Las células mononucleares de sangre periférica (PBMC) de estos pacientes fueron anérgicas a los antígenos de M. leprae en los ensayos de proliferación pero respondieron a los antígenos de las vacunas potenciales contra la lepra ( M. bovis BCG, BCG + M. leprae. y Mycobacterium w ). El enriquecimiento de las células T reactivas con M. leprae se logró estableciendo líneas de células T a partir de las PBMC estimuladas in vitro con M. leprae, M. bovis BCG, M bovis BCG + M. leprae, y Mycobacterium w . En los ensayos de proliferación, 1 de 10, 3 de 10, 6 de 10 y 2 de 10 líneas de células T establecidas contra M. leprae. M. bovis BCG, M. bovis BCG + M. leprae, y Mycobacterium w . respectivamente, respondieron a M. leprae. Estos resultados sugieren que el enriquecimiento de las células T reactivas con M. leprae preexistentes contribuye a la restauración de la respuesta de células T hacia el microorganismo en algunos de los pacientes lepromatosos. Cuatro de las 6 líneas de células T inducidas con M. leprae proliferaron en respuesta a los antígenos recombinantes de 65 kD, 36 kD, 28 kD, y 12 kD de M. leprae, sugiriendo que la falta de respuesta de las células T en algunos pacientes con lepra se puede revertir utilizando antígenos recombinantes de M. leprae.Leprosy is a chronic disease with a well-defined clinical, histopathologic and immunological spectrum. Cell-mediated immunity (CMI) plays the most important role in protection against leprosy (5). The causative agent, Mycobacterium leprae, induces a strong and long-lasting CMI response in healthy humans (6,8,26) and tuberculoid patients with self-limiting disease (5), but lepromatous leprosy patients with disseminated disease are anergic to M. leprae antigens in CMI assays (5). More recent studies demonstrate that the nonresponsiveness of lepromatous T cells to M. leprae antigens is not absolute. In vivo nonresponsiveness of T cells to M. leprae antigens has been overcome in a large proportion of lepromatous patients by immunization with candidate antileprosy vaccines, e.g., M. bovis BCG (16,32) , Mycobacterium w (2,41,44,45) and a mixture of M. bovis BCG + killed M. leprae (3,4,38). The in vitro nonresponsiveness of T cells to M. leprae can be abrogated in about 60% of lepromatous patients by providing exogenous interleukin-2 (IL-2) to T-cell cultures stimulated with M. leprae (11,13). M. leprae -reactive T cells can be demonstrated in lepromatous patients during erythema nodosum leprosum (ENL) (18) and M. leprae -specific T-cell clones have been generated from long-term-treated lepromatous patients (9). All of these studies suggest that M. leprae -reactive T cells in the lepromatous patients do exist but probably at a low frequency.
Lepromatous T cells respond to the antigens of cultivable mycobacteria which have been selected as candidate antileprosy vaccines (5,10,30). The selected mycobacteria have antigens that crossreact with M. leprae in T-cell functions, i.e., delayed-type hypersensitivity (DTH) skin response, antigen-induced proliferation, and IL-2 production (19,24,29). Since the basic defect in lepromatous T cells lies in their inability to produce IL-2 and other regulatory cytokines in response to M. leprae (11,33) one of the ways by which immunizations with candidate antileprosy vaccines might be elevating the CMI response to M. leprae is by producing IL-2 and other regulatory cytokines which enrich and expand pre-existing M. leprae -responsive T cells in lepromatous patients. Once M. leprae -reactive T cells are enriched and expanded, they may subsequently respond to M. leprae antigens upon retesting. The present study was undertaken to determine if the above proposition can be verified in vitro.
M. leprae -reactive T cells in the peripheral blood mononuclear cells (PBMC) of lepromatous patients were enriched by establishing T-cell lines against the antigens of candidate antileprosy vaccines. When tested for reactivity to M. leprae, T-cell lines from some lepromatous patients responded to M. leprae antigens in proliferative assays. The best restoration of M. leprae response was observed among T-cell lines established against M. bovis BCG + M. leprae.
MATERIALS AND METHODS
Antigens. Killed preparations of M. leprae were kindly supplied by Dr. R.J.W. Recs through the WHO/IMMLEP Bank. M. bovis BCG was obtained from Serum Institute, Copenhagen, Denmark. Killed Mycobacterium w was a kind gift from Professor G. P. Talwar, National Institute of Immunology, New Delhi, India. These mycobacteria were used at concentrations optimal forT-cell proliferation, i.e., M. leprae at 5 x 107 bacilli/ml, M. bovis BCG at 10 µ/ml (wet weight), and Mycobacterium w at 5 x 106 bacilli/ml.
Escherichia coli lysates containing 65 kilodalton (kDa), 36 kDa, 28 kDa, 18 kDa, 12 kDa (43) and 13B3 (27) recombinant antigens of M. leprae and 65 kDa, 19 kDa and 14 kDa recombinant antigens of M. tuberculosis (42) were prepared according to the protocols described earlier (20,28,34), E. coli lysates lacking recombinant antigens were used as the control. The lysates at a protein concentration of 50 µg/ml were used for T-cell proliferation assays (20,28,34).
Patients and T-cell lines. Heparinized blood was obtained from leprosy patients attending the outdoor clinic of the All Africa Leprosy Research and Training Centre, Addis Ababa, Ethiopia. The patients were classified according to the Ridley and Jopling scale (39). PBMC were separated from the heparinized blood by notation on Ficoll/Hypaque gradients (Lymphoprep). T-cell lines were established from the PBMC of leprosy patients as described earlier In brief, 2 x 106 PBMC/ml complete medium (RPMI-1640 + 15% heat inactivated AB serum + 1% pencillin-streptomycin) were stimulated with optimal concentrations of M. leprae, M. bovis BCG + M. leprae, and Mycobacterium w in the wells of 24-well costar plates (Costar, Cambridge, Massachusetts, U.S.A.). The plates were incubated for 6 days at 37°C in an atmosphere of 5% CO2 and 95% air. Thereafter, to expand the antigen-reactive T cells, recombinant IL-2 was added to the cultures at 100 U/ml twice a week. After 3-4 weeks, the T-cell lines were tested for antigen responsiveness in proliferation assays.
Proliferation assays. Assays of antigen-induced proliferation of PBMC were performed by using 1 x 105 cells/well in complete medium in 96-well, flat-bottom microtiter plates (Costar). In proliferation assays of T-cell lines, 2 x 104 T cells were added together with antigen presenting cells from 5 x 104 irradiated autologous PBMC 23). Experimental cultures were stimulated with antigens in triplicate. The control cultures did not have antigen. To test the proliferative potential of the T-cell lines, 2 x 104 cells were cultured in the presence of 10 U/ml of IL-2 alone. To assess the response against recombinant mycobacterial antigens, the experimental cultures were stimulated with E. coli lysates containing recombinant antigens. The cultures with E. coli lysates lacking recombinant antigens were taken as controls. The total culture volume was kept at 200 µl. The plates were incubated at 37°C in an atmosphere of 5% CO, and 95% air. One µCi -3H-thymidine was added to each well of the PBMC cultures on day 6 and to the cultures with T-cell lines on day 3. The plates were further incubated for 4 hr at 37°C. Cultures were harvested and the radioactivity incorporated was determined by standard methods (21). Mean counts per minute (cpm) ± standard deviation (S.D.) of the triplicate values have been used to express the results. The cells were considered responding to a given antigen when the cpm in antigen-stimulated cultures was > 500 and the Stimulation Index (SI) was > 2. Such values are underlined in the tables. The SI is defined as: SI = cpm in cultures with antigen/cpm in cultures without antigen.
RESULTS
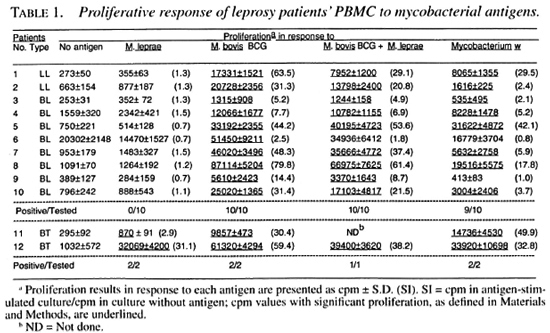
In this study, we investigated 10 Iepromatous (2 LL and 8 BL) leprosy patients whose PBMC did not show a detectable response to M. leprae antigens in proliferative assays (Table 1). Two tuberculoid (BT) leprosy patients responding to M. leprae antigens were also included for comparison purposes (Table 1). The nonresponsiveness of PBMC from the lepromatous patients was specific to M. leprae antigens because the same PBMC responded to M. bovis BCG, M. bovis BCG + M. leprae and Mycobacterium w (Table 1).
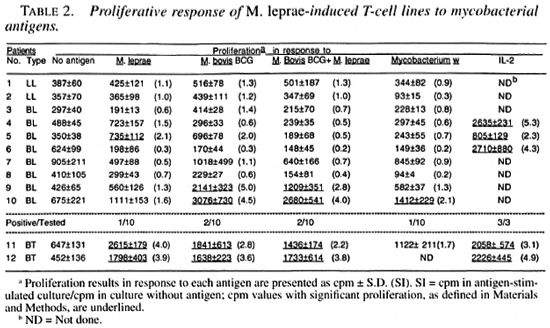
T-cell lines were established after stimulation of the patients' PBMC with M. leprae, M. bovis BCG, M. bovis BCG + M. leprae, and Mycobacterium w. All of the tested T-cell lines established against different antigens responded to recombinant IL-2 (Tables 2-5). suggesting that the T cells were capable of responding to an appropriate stimulus.
Only 1 of the 10 M. leprae -induced T-cell lines established from lepromatous patients responded to M. leprae on restimulation; whereas both of the T-cell lines from tubernonspecifically activated and expanded by culoid patients responded to M. leprae and IL-2 did not contribute much to the T-cell other mycobacterial antigens (Table 2). Most of the M. leprae -induced T-cell lines from the lepromatous patients did not respond to M. boris BCG, M. bolds BCG + M. leprae , and Mycobacterium w , suggesting that the T cells which might have been nonspecitically activated and expanded byIL-2 did not contribute much to the T-cell response driven by the specific antigens.
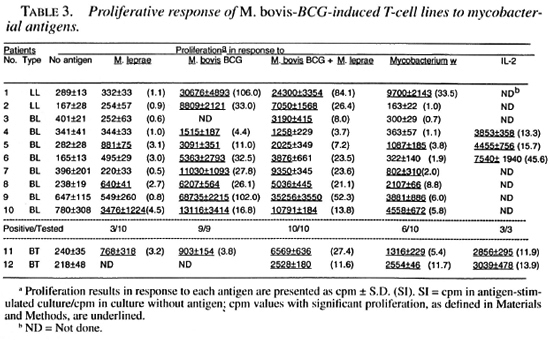
Among M. bovis BCG-induced T-cell from the lepromatous patients did not relines, 3 of the 10 T-cell lines from lepromas pond to M. bovis BCG, M. bovis BCG + tous patients responded to M. leprae; twhereas all of them responded to M. bovis ing that the T cells which might have been BCG and M. bovis BCG + M. leprae. Six of these T-cell lines responded by Mycobacterium w antigens (Table 3).
Six of the 10 T-cell lines establishedagainst M. bovis BCG + M. leprae fromlepromatous patients responded to M. leprae on restimulation (Table 4). All of the 10T-cell lines responded to M. bovis BCG and M. bovis BCG + M. leprae and seven of them responded to Mycobacterium w (Table 4).
Two of the 10 Mycobacterium w -inducedT-cell lines established from lepromatous patients responded to M. leprae antigens. Most of these T-cell lines responded to M.bovis BCG, M. bovis BCG + M. leprae , and Mycobacterium w antigens (Table 5).
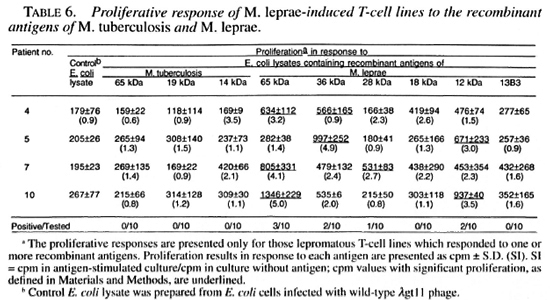
M. leprae -induced T-cell lines were alsotested for reactivity to different recombinant antigens of M. leprae and M. tuberculosis to determine if T cells capable of responding to defined antigens of mycobacte-ria existed in lepromatous patients. The 65kDa, 36 kDa, 28 kDa and 12 kDa recombinant M. leprae antigens stimulated 3, 2, I,and 2 T-cell lines, respectively (Table 6). None of the M. leprae -induced T-cell linesresponded to the 18 kDa and 13B3 recom-binant antigens of M. leprae or to the 65kDa, 19 kDa and 14 kDa recombinant antigens of M. tuberculosis (Table 6).
DISCUSSION
Our earlier studies have shown that proliferative nonresponsiveness of lepromatous T cells to M. leprae antigens is a manifestation of the inability of the T cells to produce IL-2 in response to M. leprae (11).If IL-2 is provided exogenously, M. leprae -specific proliferation can be induced in lep-romatous T cells (11,13). The in vivo relevance of these in vitro findings has been demonstrated in clinical trials with IL-2 (14,15). Injections of low doses of IL-2 into the cutaneous lesions of lepromatous patients have shown the generation of an effective CMI response, recapitulating an antigen-driven event and leading to striking local reductions in M. leprae (15). In other studies, immunizations of lepromatous leprosy patients with candidate antileprosy vaccines based on crossreactive cultivable mycobacteria either alone, e.g., M. bovis BCG (16,32) and Mycobacterium w (2,41,44,45), or along with M. leprae, i.e., M. bovis BCG + killed M. leprae (3,4,38) have shown upgrading of bacterial and immunological status similar to what has been reported by injecting IL-2. It is possible that immunizations with the mycobacteria of candidate antileprosy vaccines, which have antigens that crossreact with M. leprae in T-cell functions (19,24,29) may restore the response to M. leprae by enriching pre-existing M. leprae -responsive T cells. Since T cells from lepromatous patients are anergic to M. leprae antigens but respond to the antigens of candidate antileprosy vaccines (5,11; Table 1), IL-2 produced in response to the antigens of candidate antileprosy vaccines may enrich the T cells responsive to M. leprae antigens.
The results of this study suggest that the enrichment of pre-existing M. leprae -responsive T cells by activation with candidate antileprosy vaccines may contribute to the restoration of the M. leprae response in some lepromatous patients. Among the T-cell lines established against M. bovis BCG and Mycobacterium w, two and three T-cell lines, respectively, responded to M. leprae; whereas 6 of the 10 T-cell lines established against M. bovis BCG + M. leprae responded to M. leprae. These results could be explained on the basis that M. bovis BCG and Mycobacterium w can enrich only those M. leprae -reactive T cells which recognize crossreactive antigens; whereas the activation of PBMC with M. bovis BCG + M. leprae can also enrich the T cells responsive to M. leprae -specific antigens. Consistent with our findings, this should lead to an increased possibility of the restoration of the M. leprae response among T-cell lines established against M. bovis BCG + M. leprae as compared to the T-cell lines established against M. bovis BCG and Mycobacterium w.
Although, 3/10, 6/10 and 2/10 T-cell lines from lepromatous patients, established against M. bovis BCG, M. bovis BCG + M. leprae, and Mycobacterium w, respectively, showed positive response to M. leprae, the responses in general were low (SI range 2.1 to 9.1), especially when compared with M. bovis BCG responses (SI range 2.7 to 106). These results are comparable with what has been reported by others after immunization of lepromatous patients with M. bovis BCG + killed M. leprae (38) and Mycobacterium w (41). When tested for M. leprae -induced proliferative response of PBMC, none of the lepromatous patients showed significant proliferation (SI > 2) prior to immunizations (38-41). Following immunizations and improvements in clinical, bacteriological, histopathological and immunological (conversion to lepromin positivity) status, PBMC from 60%-70% of the patients showed significant proliferation (SI > 2) in response to M. leprae (38-41). However, the extent of the positive response to M. leprae was considerably lower [SI ranges 2 to 10 and 2 to 8.4 after immunizations with M. bovis BCG + M. leprae (38) and Mycobacterium w (41), respectively compared to the proliferation in response to M. bovis BCG [SI range 2 to 50 (38)] and purified protein derivative [SI range 3.0 to 38.3 (41)].
The addition of IL-2 to PBMC cultures stimulated with M. leprae can restore M. leprae -specific responsiveness in T cells from 60% of lepromatous patients (11,13), but in this study only 1 of the 10 M. leprae -activated and IL-2-expanded T-cell lines responded to M. leprae (Table 1). The discrepancy between the results of the present and earlier studies may be explained on the basis of the difference in the time of adding IL-2 to the cultures. In the earlier studies, IL-2 was added along with M. leprae antigens on day 0; in the present study, IL-2 was added on day 6 of antigen stimulation. IL-2-induced proliferation of T cells requires expression and up-regulation of high-affinity IL-2 receptors. Specific antigen as well as IL-2 is required for the expression and up-regulation of high-affinity IL-2 receptors on T cells (22). In earlier experiments where M. leprae and IL-2 were provided simultaneously to the T-cell cultures, antigen-induced expression of high-affinity IL-2 receptors on T cells would have been up-regulated by IL-2 and the T cells would have been triggered to proliferate by the interaction of IL-2 with high-affinity IL-2 receptors. In the present experiments, the addition of IL-2 to the cultures was delayed for 6 days and, therefore, the receptors expressed in response to M. leprae antigens would have mostly disappeared by the time exogenous IL-2 was added to the cultures. The experiments with T-cell lines established against M. bovis BCG + M. leprae will be comparable to the activation of PBMC in cultures by the simultaneous addition of M. leprae and IL-2. In these experiments, IL-2 produced in response to M. bovis BCG during the early phases of cell activation will up-regulate the high-affinity IL-2 receptors induced on T cells in response to M. leprae and drive M. leprae -specific T cells to proliferate. This will enrich M. leprae -specific T cells in addition to the enrichment of T cells responsive to crossreactive antigens. The results of this study support the above view since the M. leprae response was restored in T cells from 60% of the lepromatous patients by either establishing T-cell lines against M. bovis BCG + M. leprae or by the stimulation of PBMC with M. leprae + IL2(11,13).
As compared to only 1 of the 10 M. leprae -induced T-cell lines, established from lepromatous patients, responding to whole M. leprae, four of these T-cell lines responded to different recombinant antigens of M. leprae . The presence of antigens/epitopes activating suppressor and helper T cells have been demonstrated in M. leprae (1,37). The observed nonresponsiveness of lepromatous T cells to whole M. leprae or to total sonicates may result from the activation of suppressor cells that suppress the response of helper T cells (1,37). In experimental models of nonresponsiveness, it has been shown that the suppression mediated by complex antigens having suppressor as well as helper epitopes could be overcome by using amputated antigens having only helper epitopes (17,40). Our results suggest that a similar mechanism may also be operating in some lepromatous leprosy patients (Table 6). The restoration of the proliferative response to isolated antigens of M. leprae prepared by one- and two-dimensional gel electrophoresis has been reported, but the exact nature of the stimulating antigens was not identified (10,36). By using recombinant antigens of M. leprae, we have for the first time shown that 65 kDa, 36 kDa, 28 kDa and 12 kDa recombinant proteins possess helper T-cell epitopes capable of overcoming the possible effect of suppressor T cells in lepromatous leprosy. Since full length protein antigens may still have both helper and suppressor T-cell epitopes (31), the identification of epitopes recognized by helper T cells from lepromatous patients may be useful in designing subunit vaccine(s) against leprosy.
An interesting observation was the reactivity of T-cell lines to the M. leprae 65 kDa recombinant antigen but not to the M. tuberculosis 65 kDa recombinant antigen. Similar observations were made by Ottenhoff, et al. who have reported that lepromatous T cells responded to 61 kDa-68 kDa antigenic fractions of M. leprae prepared by one-dimensional gel electrophoresis but not to the 65 kDa recombinant antigen of M. bovis BCG (36). Ottenhoff, et al. did not test the M. leprae 65 kDa recombinant antigen but, on the basis of high amino acid sequence homology (>90%) between M. leprae and M. bovis BCG 65 kDa recombinant antigens, they suggested that the 65 kDa recombinant mycobacterial antigen was not involved in the proliferative response of lepromatous T cells. However, the existence of species-specific T-cell epitopes on the 65 kDa mycobacterial proteins has been demonstrated by testing M. leprae and M. tuberculosis 65 kDa recombinant antigen-specific T-cell clones obtained from killed M. leprae -vaccinated healthy subjects and (26,35). The results of this study suggest that the inability of lepromatous T cells to respond tothe M. bovis BCG 65 kDa recombinantantigen (37) could have been due to the M.leprae specificity of the T-cell epitopespresent on the M. leprae 65 kDa recombinant antigen.
Acknowledgment. This study was supported by the Kuwait University Research Administration Grant MI073 and by the UNDP/World Bank WHO Special Programme for Research and Training in Tropical Diseases (TDR). I am also thankful to Drs. R. Kiessling and P. J. Converse for their kind help.
REFERENCES
1. Bloom, B. and Mehra, V. Immunological unresponsiveness in leprosy. Immunol. Rev. 80(1984)5-28.
2. Chaudhuri, S., Fotedar, A. R. and Talwar, G. P. Lepromin conversion in repeatedly lepromin negative BL/LL patients after immunization with autoclaved Mycobacterium w. Int. J. Lepr. 51(1983)159-168.
3. Convit, J., Aranzazu, N., Ulrich, M., Pinardi, M. E., Reyes, O. and Alvarado, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda negative contacts. Int. J. Lepr. 50(1982)415-424.
4. Convit, J., Ulrich, M., Aranzazu, N., castellanos, P. L., Pinardi, M. E. and Reyes, O. The development of a vaccination model using two microorganisms and its application in leprosy and leishmaniasis. Lepr. Rev. 57Suppl. 2(1986) 263-273.
5. Gill, H. K. and Godal, T. Deficiency of cell-mediated immunity in leprosy. Prog. Allergy 37(1986)377-390.
6. Gill, H. K., Mustafa, A. S. and Godal, T. Induction of delayed-type hypersensitivity in human volunteers immunized with a candidate leprosy vaccine consisting of killed Mycobacterium leprae. Bull. WHO 64(1986)121-126.
7. Gill, H. K., Mustafa, A. S. and Godal, T. In vitro proliferation of lymphocytes from human volunteers vaccinated with armadillo-derived killed M. leprae. Int. J. Lepr. 55( 1987)30-35.
8. Gill, H. K., Mustafa, A. S. and Godal, T. Vaccination of human volunteers with heat-killed M. leprae: local responses in relation to the interpretation of the lepromin reaction. Int. J. Lepr. 56(1988)36-44.
9. Gill, H. K., Ridley, D. S., Ganesan, J., Mustafa, A. S., Rees, R. J. W. and Godal, T. Mycobacterium leprae reactive T cell clones from lepromatous leprosy patients after prolonged chemotherapy. Lepr. Rev. 61(1990)25-31.
10. Gulle, H., Schoel, B., Chiplunkar, S., Gangal, S., Deo, M. G. and Kaufmann, S. H. E. T-cell responses of leprosy patients and healthy contacts toward separated protein antigens of Mycobacterium leprae. Int. J. Lepr. 60(1992)44-53.
11. Haregewoin, A., Godal, T, Mustafa, A. S., Belehu, A. and Yemaneberhan, T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature 303(1983)342-344.
12. Haregewoin, A., Longely, J., Mustafa, A. S. and Godal, T. The role of interleukin-2 in the specific unresponsiveness of lepromatous leprosy to Mycobacterium leprae; studies in vivo and in vitro. Immunol. Lett. 11(1986)249-252.
13. Haregewoin, A., Mustafa, A. S., Helle, I., Waters, M. F. R., Leiker, D. L. and Godal, T. Reversal by interleukin-2 of the T-cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol. Rev. 80(1984)77-86.
14. Kaplan, G., Britton, W. J., Hancock, G. E., Theuvenet, W. J., Smith, K. A., Job, C. K., Roche, P. W., Molloy, A., Burkhardt, R.. Barker, J., Pradhan, H. R. and Cohn, Z. A. The systemic influence of recombinant interleukin-2 on the manifestations of lepromatous leprosy. J. Exp. Med. 173(1991)993-1006.
15. Kaplan, G., Kiessling, R. Teklemariam, S., Hancock, G., Sheftel, G., Job, C. K., Converse, P., Otthnhoft, T. H. M., Becx-Bleumink, M., Deetz, M. and Cohn, Z. A. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin-2. J. Exp. Med. 169(1989)893-907.
16. Katoch, K., Natarajan, M., Bagga, A. K.. Narayana, R. B. and Katoch, V. M. Immunotherapy of treated BL/LL cases with BCG: histopathological, immuhistological and bacteriological assessment. Acta Leprol. 7 Suppl. 1(1989)153-155.
17. Krzych, U., Fowler, A. V. and Sercarz, E. E. Repertoires of T cells directed against a large protein antigen, beta galactosidase. II. Only certain T helper or T suppressor cells are relevant in particular regulatory interactions. J. Exp. Med. 162(1985)311-323.
18. Laal, S., Bhutani, L. K. and Nath, I. Natural emergence of antigen-reactive T cells in lepromatous leprosy patients during erythema nodosum leprosum. Infect. Immun. 50(1985)887-892.
19. Mustafa, A. S. Identification of T-cell activating recombinant antigens shared among three candidate antileprosy vaccines, killed M. leprae, M. bovis BCG and Mycobacterium w. Int. J. Lepr. 50(1988)265-273.
20. Mustafa, A. S., Gill, H. K., Nerland, A., Britton, W. J., Mehra, V., Bloom, B. R., Young, R. A. and Godal T. Human T cell clones recognize a major M. leprae antigen expressed in E. coli. Nature (London) 319(1986)63-66.
21. Mustafa, A. S. and Godal, T. In vitro introduction of human suppressor T cells by mycobacterial agents. BCG activated OKT4+ cells mediate suppression of antigen-induced T cell proliferation. 33.Clin. Exp. Immunol. 52(1983)29-37.
22. Mustafa, A. S. and Godal T. BCG induced suppressor T cells optimal conditions for in vitro induction and mode of action. Clin. Exp. Immunol. 62(1985)474-481.
23. Mustafa, A. S. and Godal, T. BCG induced CD4+ cytotoxic T cells from BCG vaccinated 34.healthy subjects: relation between cytotoxicity and suppression in vitro. Clin. Exp. Immunol. 69(1987)255-262.
24. Mustafa, A. S., Kvalheim, G., Degre, M. and Godal, T. Mycobacterium bovis BCG induced human T cell clones from BCG vaccinated healthy subjects: antigen specificity and lymphokine production. Infect. Immun. 53(1986)491-497.
25. Mustafa, A. S., Lundin, K. E. A. and Oftung, F. Human T cells recognize mycobacterial heat shock proteins in the context of multiple HLA-DR molecules: studies with healthy subjects vaccinated with Mycobacterium bovis BCG and Mycobacterium leprae. Infect. Immun. 61(1993)5294-5301.
26. Mustafa, A. S. and Oftung, F. Long lasting T cell reactivity to Mycobacterium leprae antigens in human volunteers vaccinated with killed M. leprae. Vaccine 11(1993)1108-1112.
27. Mustafa, A. S., Oftung, F., Deggerdal, A., Gill, H. K., Young, R. A. and Godal, T. Gene isolation with human T lymphocyte probes. Isolation of a gene that expresses an epitope recognized by T cell specific for Mycobacterium bovis BCG and pathogenic mycobacteria. J. Immunol. 141(1988)2729-2733.
28. Mustafa, A. S., Oftung, F., Gill, H. K. and Natvig, I. Characteristics of human T cell clones from BCG and killed M. leprae vaccinated subjects and tuberculosis patients. Lepr. Rev. 57Suppl. 2(1986)123-130.
29. Mustafa, A. S. and Talwar, G. R Delayed hypersensitivity skin reactions to homologous and heterologous antigens in guinea-pigs immunized with M. leprae and four other selected cultivable mycobacterial strains. Lepr. India 50(1978)509-519.
30. Mustafa, A. S. and Talwar, G. P. Early and late reactions in tuberculoid and lepromatous leprosy patients with lepromins from Mycobacteria leprae and five selected cultivable mycobacteria. Lepr. India 50(1978)566-571.
31. Mutis, T., Cornelisse, Y. E., Datema, G., Van Den Elsen, P. J., Ottenhoff, T. H. M. and Devries, R. R. P. Definition of a human suppressor T cell epitope. Proc. Natl. Acad. Sci. U.S.A.91(1994)9456-9460.
32. Natarajan, M., Katoch, K. Gabba, A. K. and Katoch, V. M. Histological changes with combined chemotherapy and immunotherapy in highly bacillated lepromatous leprosy. Acta Leprol. 8(1992)79-86.
33. Nogueira, N., Kaplan, G., Levy, E., Sarno, E. N., Kushner, P., Granelli-Piperno, A., Vieira, L., Colomer-Gould, V, Levis, W. R., Stfinman, R., Yip Y. K. and Cohn, Z. A. Defective γ-interferon production in leprosy; reversal with antigen and interleukin-2. J. Exp. Med. 158(1983)2165-2170.
34. Oftung, F., Mustafa, A. S., Husson, R., Young, R. A. and Godal, T. Human T cell clones recognize two abundant Mycobacterium tuberculosis protein antigens expressed in Escherichia coli. J. Immunol. 138(1987)927-931.
35. Oftung, F., Mustafa, A. S., Shinnick, T. M., Houghton, R. A., Valheim, G., Degre, M., Lundin, K. E. A. and Godal, T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J. Immunol. 141(1988)2749-2754.
36. Ottenhoff, T. H. M., Converse, P. J., Gebre, N., Wondimu, A., Ehrenberg, J. P. and Kiessling, R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous non-responder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur. J. Immunol. 19(1989)707-713.
37. Ottenhoff, T. H. M., Elferink, D. G., Klatser, P. R. and DE Vries, R. R. P. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. Nature 322(1986)462-464.
38. Rada, E., Ulrich, M., Aranzazu, N., Santaella, C, Gallinoto, M.. Centeno, M., Rodriguez, V and Convit, J. A longitudinal study of immuno logical reactivity in leprosy patients treated with immunotherapy. Int. J. Lepr. 62(1994)552-558.
39. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966) 255-273.
40. Wicker, L. S., Katz, M., Sercarz, E. E. and Miller, A. Immunodominant protein epitopes. I. Induction of suppression to hen egg white lysozyme is obliterated by removal of the first three N-terminal amino acids. Eur. J. Immunol. 14(1984 )442-447.
41. Yadav, A., Suresh, N. R., Zaheer, S. A., Talwar, G. P. and Mukherjee, R. T cell responses to fractionated antigens of Mycobacterium w , a candidate anti-leprosy vaccine, in leprosy patients. Scand. J. Immunol. 34(1991)23-31.
42. Young. R. A., Bloom, B. R., Grosskinsky, C. M., Ivanyi, J., Thomas, D. and Davis, R. W. Detection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc. Natl. Acad. Sci. U.S.A. 82(1985) 2583-2587.
43. Young, R. A., Mehra, V., Sweetser, D., Buchanan, T., Clark-Curtiss, J., Davis, R. W. and Bloom, B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature (London) 316 (1985) 450-452.
44. Zaheer, S. A., Mukherjee, A., Ramesh, V., Misra, R. S., Kar, H. K., Sharma, A. K., Beena, K. R., Walia, R., Mukherjee, R., Kaur, H. and Tamar, G. P. Imumnotherapy benefits multibacillary leprosy patients with persistently high bacteriological index despite long-term multidrug therapy. Immunol. Infect. Dis. 5(1995)115-122.
45. Zaheer, S. A., Mukherjee R., Ramkumar, B.,Misra, R. S., Sharma, A. K., Kar, H. K., Kaur, H., Nair, S., Mukherjee, A. and Tawar, G. P.Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J. Infect. Dis. 167(1993)401-410.
1. Ph.D., Associate Professor, Department of Microbiology, Faculty of Medicine, Kuwait University, P.O. Box 24923, 13110 Safat, Kuwait. FAX =965-531-8454.
Received tor publication on 28 August 1995.
Accepted for publication in revised form on 28 February 1996.