- Volume 64 , Number 3
- Page: 327–9
Antibodies to phenolic glycolipid-l and sulfatide-l in leprosy and tuberculosis
To the Editor:
Following the discovery of phenolic glycolipid-I (PGL-I) by Hunter and Brennan (11) in Mycobacterium leprae -infected tissues and once being established that the material was specific to M. leprae (12), several assays for the detection of that lipid antigen have been developed with the intention of applying them in the serological diagnosis of leprosy (5,18) to identify those household contacts with an incipient disease (4) and to monitor the response of the patients subjected to chemotherapy (2). A similar glycolipid has been isolated and characterized from M. tuberculosis by Daffe, et al. (7) and has been used by some authors for the serological diagnosis of tuberculosis with variable results (3,15,16).
In this study, we measured the reactivity of the sera from 34 tuberculous patients, 33 patients with lepromatous leprosy, and 38 healthy individuals to PGL-I of M. leprae and to sulfolipid-I of M. tuberculosis H37Rv. Each lipid has been considered to be species-specific, and in the case of PGL-I, this specificity has been the basis for its use as an antigen for the serological diagnosis of leprosy. Although a similar consideration of specificity has been given to the sulfolipid-I (sulfatide-I, SL-I) of M. tuberculosis, its use as an antigen for the diagnosis of tuberculosis has not been a common practice, perhaps because of the more extensive information on protein antigens (1,9,14) and other lipids (6,8). PGL-I was isolated from M. leprae- infected armadillo tissue by the techniques of Vemuri, et al. (17) and Hunter, et al. (13). SL-I was purified from M. tuberculosis H37Rv using the method of Goren, et al. (10). Although the patients studied were under treatment at the time of sampling and most leprosy patients were old multitreated cases, all of the patients still had active disease. Patients and control groups included both male and female individuals whose ages ranged from 16 to 72 years.
Antibodies to the mycobacterial lipids were measured using an enzyme-linked immunosorbent assay (ELISA) adapted for lipid antigens. From the results, it could be concluded that: a) lepromatous (LL) sera and tuberculous (Tb) sera contain similar amounts of IgG antibodies to PGL-I [0.154 ±0.101 (mean OD 492 nm of triplicates ± S.D.) in LL vs 0.104 ± 0.052 in Tb, p = 0.5J; b) LL sera contain higher levels of IgM antibodies to PGL-I than Tb sera (0.164 ± 0.227 vs 0.046 ± 0.035, respectively; p = 0.01); c) LL sera and Tb sera show similar amounts of IgG antibodies to SL-I (0.144 ± 0.072 vs 0.096 ± 0.050, p = 0.5); d) LL sera and Tb sera contain similar, very low amounts, if any, of IgM antibodies to SL-I (0.019 ± 0.034 vs 0.026 ± 0.020, respectively; p = 0.5), and e) although low, the levels of IgG and IgM antibodies to PGL-I and to SL-I in LL and Tb sera were still higher than those levels in the control group, with the numerical values not always reaching statistical significance.
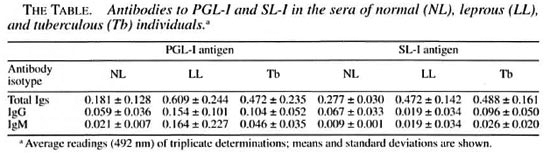
These results may indicate a) that M. leprae contain antigens with epitopes related to SL-I, b) that some antigen(s) in M. tuberculosis may share one or more epitopes with the PGL-I of M. leprae, or c) that leprosy patients are (or were) also infected by M. tuberculosis, with this infection not necessarily overt but subclinical. The third possibility seems to be more likely since antibodies to SL-I were also found in several healthy subjects, a fact that indicates a previous or present contact with the microorganism (in Mexico, tuberculosis is endemic and a high portion of the population is PPD+).
Taken together, the results show that a variable degree of crossreactivity with PGL-I and SL-I is detected in the serum of patients with leprosy and/or tuberculosis. This makes the use of these lipid antigens inadequate for the differential diagnosis of these mycobacterioses (more so in geographic areas where one or the two mycobacterioses are endemic).
- Julieta Luna-Herrera, Sc.Dr.
Patricia Arce-Paredes, Sc.B.
Oscar Rojas-Espinosa, Sc.Dr.
Departmento de Inmunología
Escuela Nacional de Ciencias Biológicas
Instituto Politécnico Nacional
Carpio y Plan de Avala
Colonia Santo Tomas
11340 Mexico, D.F., Mexico.
Acknowledgment. This study received financial support from the Dirección de Estudios de Posgrado e Investigation del IPN (Proyectos 933635 and 952281). Julieta Luna was a fellow of CONACYT, Mexico; Patricia Arce is a fellow-holder of COFAA (IPN) and EDD (IPN); Osear Rojas-Espinosa is a fellow of COFAA (IPN), EDD (IPN), and SNI (Mexico).
REFERENCES
1. Andersen, P., Askgaard, D., Ljungqvist, L., Bennedsen, J. and Heron, I. Proteins released from Mycobacterium tuberculosis during growth. Infect. Immun. 59(1991)1905-1910.
2. Bach, M.-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-l and to whole M. leprae in leprosy patients; evolution during therapy. Int. J. Lepr. 54(1986)256-267.
3. Chanteau, S., Glaziou, P. and Chansin, R. Assessment of the diagnostic value of the native PGLTBL, its synthetic neoglycoconjugate PGLTB0 and the sulfolipid IV antigens for the serodiagnosis of tuberculosis. Int. J. Lepr. 60(1992)1-7.
4. Cho, S.-N., Shin, J. S., Choi, I. H., Kim, S. H., Kim, D. I. and Kim, J. D. Detection of phenolic glycolipid I of Mycobacterium leprae and antibodies to the antigen in sera from leprosy patients and their contacts. Yonsei Med. J. 29(1988)219-224.
5. Cho, S.-N., Yanaghira, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
6. Cruaud, P., Yamashita, J. T, Martin-Casabona, N., Papa, F. and David, H. L. Evaluation of a novel 2,3-diacyltrehalose-21-sulfate (SL-IV) antigen for case finding and diagnosis of leprosy and tuberculosis. Res. Microbiol. 141(1990)679-694.
7. Daffe, M., Lacave, C, Lanelle, M. A. and Lanelle, G. Structure of major triglycosylphenolphtiocerol of Mycobacterium tuberculosis (strain Canetti). Eur. J. Biochem. 167(1987)155-160.
8. Daffe, M., Papa, F., Laszlo, A. and David, H. L. Glycolipids of recent clinical isolates of Mycobacterium tuberculosis: chemical characterization and immunoreactivity. J. Gen. Microbiol. 135(1989)2759-2766.
9. Daniel, T. M. Rapid diagnosis of tuberculosis: laboratory techniques applicable in developing countries. Rev. Infect. Dis. 11Suppl (1989)471-478.
10. Goren, M. B., Broke, O., Roller, P., Fales, H. M. and Das. B. S. Sulfatides of Mycobacterium tuberculosis; the structure of the principal sulfatide (SLI).Biochemistry 15(1976)2728-2735.
11. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
12. Hunter, S. W., Fujiwara, T. and Brennan, P. J.Structure and antigenicity of the major specific glycolipid specific for My cobacterium leprae J.Biol. Chem. 257(1982)15072-15078.
13. Hunter, S. W., Stewart, C. and Brennan, P. J. Purification of phenolic glycolipid I from armadillo and human sources. Int. J. Lepr. 53(1985)484-486.
14. Nagai, S., Wiker, H. G., Harboe, M. and Kinomoto, M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Immun. 59(1991)372-382.
15. Torgal-Garcia, J., David, H. L. and Papa, F. Preliminary evaluation of a Mycobacterium tuberculosis phenolglycolipid antigen in the serological diagnosis of tuberculosis. Ann. Inst. Pasteur/Mcrobiol. 139(1988)289-294.
16. Torgal-Garcia, J., Papa, F. and David, H. L. The use of PGL-TB1 from M. tuberculosis in the serological diagnosis of tuberculous meningitis: a preliminary report. Acta Leprol. 7 Suppl.(1989)136.
17. Vemuri, N., Kmandke, L.. Mahadevan, P. R. Hunter, S. W. and Brennan, P.J. Isolation of phenolic glycolipid-I from human lepromatous nodules. Int. J. Lepr. 53(1985)487-489.
18. Young, D. B. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific of Mycobacterium leprae . Science 221(1983)1057-1059.
Reprint requests to Dr. Rojas-Espinosa.