- Volume 64 , Number 3
- Page: 329–32
NADPH-Oxidase activity triggered by endocytosis of yeast in circulating phagocytes of nine-banded armadillos (dasypus novemcinctus)
To the Editor:
Dasypus novemcinctus, the nine-banded armadillo, is regarded as a host for Mycobacterium leprae (7,8,12). In those geographic areas where leprosy is endemic, wild armadillos have been considered potential natural reservoirs for the leprosy bacillus (10). Despite its great importance as an alternative host for M. leprae, the immunobiology of the armadillo is an aspect still little explored. As in humans, armadillo M. leprae is also an intracellular parasite of macrophages; M. leprae- laden macrophages accumulate to form macrophagic granulomas, the characteristic lesions of leprosy. Granulomas may appear internally in the viscerae and more superficially in the skin and underlaying structures (1). The fate of M. leprae in the armadillo is very likely dependent on the function of macrophages (modulated by cell-mediated immunity), and early in the infection it is probably also dependent on the function of polymorphonuclear (PMN) leukocytes. Macrophages and PMN leukocytes are professional phagocytic cells of the body whose combined function accounts for the killing of invading microorganisms; killing by phagocytic cells depends, in turn, on a variety of mechanisms of which some are oxygen-dependent and some are not (11). Oxygen-dependent mechanisms include the generation of highly reactive oxygen species (free radicals) as well as the activity of the myeloperoxidase system (9). Among the microbicidal mechanisms that do not depend on oxygen are the acidic milieu within the phagolysosomes, the hydrolytic activity of neutral and acidic hydrolases, the effect of cationic proteins other than hydrolytic enzymes, and the activity of factors that increase the permeability of bacterial cell walls (BPIFs). An additional microbicidal mechanism, this one strongly exerted by immunologically activated macrophages, depends on the metabolism of arginine in which nitric oxide is an important byproduct (5). As oxygen metabolites (superoxide anion, hydroxyl radicals and oxygen singlet), nitric oxide is a highly unstable species and thus an extremely reactive radical. Oxygen metabolites and nitric oxide are able to chemically modify the microbial body, arresting its metabolism, leaving the microorganism inert and unable to repair itself, and exposed to the digestive action of the acid and neutral hydrolases found within the phagolysosome.
Stimulation of phagocytes via receptors to chemotactic factors, or opsonins, induces changes in the cell membrane whose signals are transducted to the cytoplasm and nucleus of the cell, leading to specific and more general biochemical changes. These changes are diverse but include increment in the cell activities related to phagocytosis (chemotaxis, endocytosis, and microbial killing) (6). C3b-opsonized yeast interacts with CR1 receptors on the cell membrane in a manner regulated by G-protcins to next activate an effector molecule or second messenger (4). When the effector is an enzyme active on membrane inositol-phospholipids (a phospholipase C, for instance), the hydrolizing activity of the enzyme releases inositol triphosphate (IP3) and diacyl glycerol (DAG). While the role of IP3 has much to do with Ca+ + mobilization through the opening of Ca++channels, DAG interacts with and activates a type of protein kinase C (PKC). In one particular situation, the activated PKC translocates to the inner part of the cell membrane to interact with the NADPH-oxidase system (2,3). The NADPHoxidase system involves a trimolecular complex (proteins p47, p67 and Cyt B558) whose function is the trapping and chemical transformation of oxygen to more reduced species. Oxygen is first reduced to superoxide anion (O-2) which is then transformed, either spontaneously or by the action of the enzyme superoxide dismutase (SOD), to hydrogen peroxide (H2O2). Superoxide anion may react with hydrogen peroxide to produce hydroxyl radicals (OH-) or with extra molecules of superoxide anion to produce the so-called oxygen singlet (102). Superoxide, hydrogen peroxide, hydroxyl radicals, and oxygen singlet constitute the oxygen-dependent microbicidal armory of phagocytic cells. Apart from oxygen, activated NADPH-oxidase may use other (artificial) electron acceptors such as nitro blue tetrazolium or NBT (2,2'-di-p-nitrophenyl-5,5'-diphenyl-3,3'-[3,3'-dimethoxy-4,4'diphenylene]-ditetrazolium chloride). Oxidized NBT is reduced to NBTH2, a blue formazan that precipitates at the site where it is reduced. This is the basis for the "NBT test" frequently used to assess the bactericidal activity of human phagocytic cells.
We performed the NBT test in the circulating leukocytes of two nine-banded armadillos in order to explore whether or not this animal species possesses and makes usethe NADPH-oxidase system to generate microbicidal metabolites in response to phagocytic stimulation. Armadillos (Dasypus noveincinctus) were captured in Sinaloa, a state in western Mexico bordering on the Gulf of California, and sent by air to Mexico City where the studies were performed.
Experimental. Ten ml of blood was taken by heart puncture from two armadillos maintained under light anesthesia with chloroform, and was immediately transferred to silicon-treated, screw-cap, glass tubes containing 12 glass beads (3-mm diameter) each. The tubes were capped and their contents mixed by repeated inversion of the tubes to promote blood defibrination (about 5 min), then 0.5 ml of the fibrin-free blood was deposited on each of several cov-erslips previously degreased, washed, and sterilized. Leukocytes were allowed to adhere to the slides for 30 min at 37ºC under 5% CO2 in a moist atmosphere. Meanwhile, 1.0 ml of heat-killed baking yeast, previously prepared and adjusted to 300 x 106 cells per ml in saline solution, were washed and opsonized with fresh autologous (armadillo) serum or serum previously inactivated at 56ºC for 30 min. To opsonize yeast, 1.0 ml of the suspension was centrifuged at 600 x g x 5 min, the pellet suspended in 1.0 ml of fresh or heat-inactivated serum, the suspended suspension incubated at 37ºC for 30 min, centrifuged as before, and finally resuspended in 1.0 ml of MEM tissue culture medium (MEM TCM).
At the end of the 30-min incubation, the coverslips containing the defibrinated blood were picked up, gently rinsed twice in MEM TCM to eliminate most erythrocytes, and then transferred to plastic petri dishes (2.5-cm diameter) containing 2 ml of MEM TCM, 30 x 106 yeast, and 0.5 ml of a 0.10% NBT solution in 0.15 M NaCl (a couple of cell-containing coverslips were stained with Giemsa's stain to make a differential count). The dishes were incubated for a further 30 min, the coverslips were then removed, rinsed 3 times in physiological saline, stained for 10 min with 0.5% safranin in water, rinsed in distilled water, air dried, and mounted on glass slides using synthetic resin. The degree of endocytosis and NBT reduction was microscopically assessed (x500) by counting 200 cells in duplicate preparations. Blood leukocytes roughly consist of 60% PfvlN leukocytes, 30% lymphocyte-like and 10% monocyte-like cells.
We found that while 80.7% (armadillo 1) and 80.9% (armadillo 2) of the cells ingested yeast preincubated with heat-inactivated serum (nonopsonized yeast), all of the cells (100%) in both armadillos were able to ingest yeast opsonized with fresh serum. A more significant difference was based on the number of cells that ingested a maximal number of yeast: while most of the cells (over 20% in both armadillos) ingested between 2 and 3 nonopsonized yeast, most cells (nearly 50%) ingested between 6 and 10 opsonized yeast (Figs. 1 and 2). Thus, opsonization clearly increases the endocytic event. On the other hand, NBT reduction was seen in all cells that ingested yeast (100%), whether or not they were opsonized (Fig. 3).
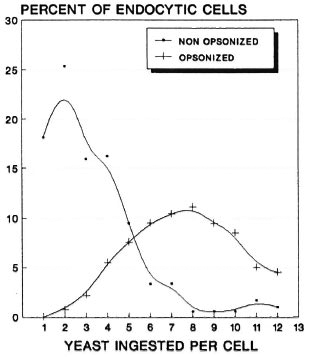
Fig. 1 Endocytic behavior of the phagocytes of ar-madillo no. 1 stimulated with nonopsonized or op-sonized yeast. While most cells ingested a small number of nonopsonized yeast (average peak of 2 yeast percell), the majority of cells ingested a larger number ofopsonized yeast (average peak of 9 yeast per cell). Percent values were calculated from 200 cells counted oneach of duplicate slides.
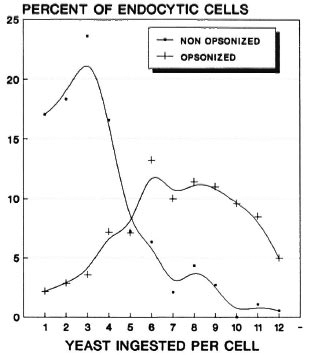
Fig. 2. Endocytic behavior of the phagocytes ofarmadillo no. 2 stimulated with nonopsonized or opsonized yeast. While most cells ingested a small number of nonopsonized yeast (average peak of 3 yeast percell), the majority of cells ingested a larger number ofopsonized yeast (average peak between 6 and 10 yeastper cell). Percent values were calculated from 200cells counted on each of duplicate slides.
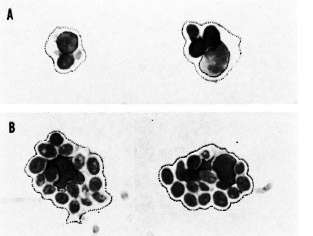
Fig. 3. Armadillo phagocytes ingesting nonop-sonized (panel A) or opsonized (panel B) yeast. Al-though there is a clear difference in the number ofyeast ingested per cell (much higher in panel B), reduced NOT is observed (dark granules or dark "staining" of yeast) in all phagocytizing cells whether yeastwas opsonized with fresh or heat-inactivated autolo-gous serum (x1200).
These results indicate that a) armadillos contain circulating phagocytes that morphologically correspond to PMN leukocytes (about 60%) and monocytic (around 10%) cells; b) armadillo phagocytic cells exhibit receptors for at least one opsonizing component (functionally analogous to C3 of other species) present in fresh serum and activable by yeast through a mechanism probably analogous to the alternative pathway of complement activation described in other species; and c) armadillo circulating phagocytic cells seem to possess the free radical-generating NADPH oxidase system through which NBT is also reduced. Possession of this system enables phagocytic cells of most species to efficiently cope with invading microorganisms. Given the long-lasting relationship between mycobacteria and phagocytic cells in leprosy, it would be very interesting to know whether or not armadillo PMN leukocytes and macrophages use this mechanism to defend themselves against M. leprae. Production of authentic superoxide anion (0-2) and H2O2 by armadillo PMN leukocytes and peritoneal and alveolar macrophages is a subject presently under investigation in our laboratory.
- Oscar Rojas-Espinosa, Sc.D.
Sonia Valderrama-Godoy, Sc.B.
Patricia Arce-Paredes, Sc.B.
Department of Immunology
Escuela Nacional de Ciencias Biológicas, 1PN
Politecnico Nacional
Carpio y Plan de Ayala
Col. Santo Tomas
11340 Mexico, DE, Mexico
- Alejandra Vazquez-Trujillo, Sc.B.
Centro de Ciencias de Sinaloa
Culiacan, Sinaloa, Mexico
Acknowledgment. This study was supported in part by the Dirección de Estudios de Posgrado e Investigación (DEPI) del I.P.N., México. O. Rojas-Espinosa holds fellowships from I.P.N. (COFAA and EDD), and SNI. México; P. Arce-Paredes is a fellow of COFAA and EDD, I.P.N.
REFERENCES
1. Binford, C. H, Storrs, E. E. and Walsh, G. P. Disseminated infection in the nine-banded armadillo (Dasypus novemcinctus) resulting from inoculation with M. leprae; observations made on 15 animals studied at autopsy. Int. J. Lepr. 44(1976)80-83.
2. Christiansen, N.O. and Borregaard, N. Translocation of protein kinase C to subcellular fractions of human neutrophils. Scand. J. Immunol. 29(1989)409-416.
3. Dieter, P. Relationship between intracellular pH changes, activation of protein kinase C and NADPH oxidase in macrophages. FEBS Lett. 298 (1992) 17-20.
4. Exton, J. H. Mechanisms of action of calcium-mobilizing agonists: some variations on a young theme. FASEB J. 2(1988)2670-2676.
5. Green, S. J., Nancy, C. A., and Meltzer, M. S. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J. Leuk. Biol. 50(1991)90-103.
6. Johnston, R. B. and Kitagawa, S. Molecular basis for the enhanced respiratory burst of activated macrophages. Federation Proc. 44(1985)2927-2932.
7. Kirchheimer, W. F. and Storrs, E. E. Attempts to establish the armadillo ( Dasypus novemcinctus. Linn) as a model for the study of leprosy. Int. J. Lepr. 39(1971)693-702.
8. Kirchheimer, W. F., Storrs, E. E. and Binford,C. H. Attempts to establish the armadillo (Dasypus novemcinctus. Linn) as a model for the study of leprosy. II. Histopathologic and bactériologie post-mortem findings in lepromatoid leprosy in the armadillo. Int. J. Lepr. 40(1972)229-242.
9. Klebanoff, S. J. Myeloperoxidasc-mediated antimicrobial systems and their role in leukocyte function. In: Biochemistry of the Phagocytic Process. Schultz, G. J., ed. Amsterdam: North Holland, 1970, pp. 89-110.
10. Meyers, W. M., Walsh G. P., Binford, C. H, Storrs, E. E. and Brown, H. L. Indigenous leprosy in nine-banded armadillos. In: The Armadillo as an Experimental Model in Biomedical Research. Washington, DC: Pan American Health Organization, 1978, pp. 67-76. Sci. Publ. No. 366.
11. Root, R. K. and Cohen, M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev. Infect. Dis. 3(1981)565-598.
12. Walsh, G. P. Experimental leprosy in the nine-banded armadillo (Dasypus novemcinctus). In: The Armadillo as an Experimental Model in Momedical Research. Washington, DC: Pan American Health Organization, 1978, pp. 57-61. Sci. Publ. No. 366.